Abstract
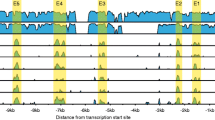
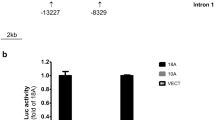
Tyrosine hydroxylase (TH) is the rate-limiting enzyme in catecholamine biosynthesis and its gene proximal promoter (< 1 kb upstream from the transcription start site) is essential for regulating transcription in both the developing and adult nervous systems. Several putative regulatory elements within the TH proximal promoter have been reported, but evolutionary conservation of these elements has not been thoroughly investigated. Since many vertebrate species are used to model development, function and disorders of human catecholaminergic neurons, identifying evolutionarily conserved transcription regulatory mechanisms is a high priority. In this study, we align TH proximal promoter nucleotide sequences from several vertebrate species to identify evolutionarily conserved motifs. This analysis identified three elements (a TATA box, cyclic AMP response element (CRE) and a 5′-GGTGG-3′ site) that constitute the core of an ancient vertebrate TH promoter. Focusing on only eutherian mammals, two regions of high conservation within the proximal promoter were identified: a ∼250 bp region adjacent to the transcription start site and a ∼85 bp region located approximately 350 bp further upstream. Within both regions, conservation of previously reported cis-regulatory motifs and human single nucleotide variants was evaluated. Transcription reporter assays in a TH -expressing cell line demonstrated the functionality of highly conserved motifs in the proximal promoter regions and electromobility shift assays showed that brain-region specific complexes assemble on these motifs. These studies also identified a non-canonical CRE binding (CREB) protein recognition element in the proximal promoter. Together, these studies provide a detailed analysis of evolutionary conservation within the TH promoter and identify potential cis-regulatory motifs that underlie a core set of regulatory mechanisms in mammals.
Similar content being viewed by others
References
Akiba N, Jo S, Akiba Y, Baker H, Cave JW (2009). Expression of EGR-1 in a subset of olfactory bulb dopaminergic cells. J Mol Histol, 40 (2): 151–155
Baker H, Kawano T, Margolis F L, Joh T H (1983). Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci, 3(1): 69–78
Baker H, Liu N, Chun H S, Saino S, Berlin R, Volpe B, Son J H (2001). Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci, 21(21): 8505–8513
Baker H, Morel K, Stone D M, Maruniak J A (1993). Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res, 614(1–2): 109–116
Barrett T J, Vedeckis W V (1996). Occupancy and composition of proteins bound to the AP-1 sites in the glucocorticoid receptor and cjun promoters after glucocorticoid treatment and in different cell types. Recept Signal Transduct, 6(3–4): 179–193
Best J A, Chen Y, Piech K M, Tank A W (1995). The response of the tyrosine hydroxylase gene to cyclic AMP is mediated by two cyclic AMP-response elements. J Neurochem, 65(5): 1934–1943
Brooks T A, Hurley L H (2009). The role of supercoiling in transcriptional control of MYC and its importance in molecular therapeutics. Nat Rev Cancer, 9(12): 849–861
Brudno M, Do C B, Cooper G M, Kim M F, Davydov E, Green E D, Sidow A, Batzoglou S, and the NISC Comparative Sequencing Program (2003). LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res, 13(4): 721–731
Burbach J P, Smits S, Smidt M P (2003). Transcription factors in the development of midbrain dopamine neurons. Ann N Y Acad Sci, 991(1): 61–68
Byrd C A (2000). Deafferentation-induced changes in the olfactory bulb of adult zebrafish. Brain Res, 866(1–2): 92–100
Cambi F, Fung B, Chikaraishi D (1989). 5′ flanking DNA sequences direct cell-specific expression of rat tyrosine hydroxylase. J Neurochem, 53(5): 1656–1659
Cave J W, Akiba Y, Banerjee K, Bhosle S, Berlin R, Baker H (2010). Differential regulation of dopaminergic gene expression by Er81. J Neurosci, 30(13): 4717–4724
Cave JW, Baker H (2009). Dopamine systems in the forebrain. Adv Exp Med Biol, 651: 15–35
Cazorla P, Smidt M P, O’Malley K L, Burbach J P (2000). A response element for the homeodomain transcription factor Ptx3 in the tyrosine hydroxylase gene promoter. J Neurochem, 74(5): 1829–1837
Chen Y C, Priyadarshini M, Panula P (2009). Complementary developmental expression of the two tyrosine hydroxylase transcripts in zebrafish. Histochem Cell Biol, 132(4): 375–381
Dawson S J, Yoon S O, Chikaraishi D M, Lillycrop K A, Latchman D S (1994). The Oct-2 transcription factor represses tyrosine hydroxylase expression via a heptamer TAATGARAT-like motif in the gene promoter. Nucleic Acids Res, 22(6): 1023–1028
Dickson P W, Briggs G D (2013). Tyrosine hydroxylase: regulation by feedback inhibition and phosphorylation. Adv Pharmacol, 68: 13–21
Filippi A, Mahler J, Schweitzer J, Driever W (2010). Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J Comp Neurol, 518(4): 423–438
Frazer K A, Pachter L, Poliakov A, Rubin E M, Dubchak I (2004). VISTA: computational tools for comparative genomics. Nucleic Acids Res, 32(Web Server issue): W273–9
Goridis C, Rohrer H (2002). Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci, 3(7): 531–541
Hack M A, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo P M, Götz M (2005). Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci, 8(7): 865–872
Hebert M A, Serova L I, Sabban E L (2005). Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem, 95(2): 484–498
Hwang D Y, Ardayfio P, Kang U J, Semina E V, Kim K S (2003). Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res, 114(2): 123–131
Ichikawa H, Mo Z, Xiang M, Sugimoto T (2005). Brn-3a deficiency increases tyrosine hydroxylase-immunoreactive neurons in the dorsal root ganglion. Brain Res, 1036(1–2): 192–195
Iwawaki T, Kohno K, Kobayashi K (2000). Identification of a potential nurr1 response element that activates the tyrosine hydroxylase gene promoter in cultured cells. Biochem Biophys Res Commun, 274(3): 590–595
Jacobs F M, van Erp S, van der Linden A J, von Oerthel L, Burbach J P, Smidt M P (2009). Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development, 136(4): 531–540
Kessler M A, Yang M, Gollomp K L, Jin H, Iacovitti L (2003). The human tyrosine hydroxylase gene promoter. Brain Res Mol Brain Res, 112(1–2): 8–23
Kim K S, Kim C H, Hwang D Y, Seo H, Chung S, Hong S J, Lim J K, Anderson T, Isacson O (2003a). Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem, 85(3): 622–634
Kim S M, Yang J W, Park M J, Lee J K, Kim S U, Lee Y S, Lee M A (2006). Regulation of human tyrosine hydroxylase gene by neuronrestrictive silencer factor. Biochem Biophys Res Commun, 346(2): 426–435
Kim T E, Park M J, Choi E J, Lee H S, Lee S H, Yoon S H, Oh C K, Lee B J, Kim S U, Lee Y S, Lee M A (2003b). Cloning and cell typespecific regulation of the human tyrosine hydroxylase gene promoter. Biochem Biophys Res Commun, 312(4): 1123–1131
Kobayashi K, Kaneda N, Ichinose H, Kishi F, Nakazawa A, Kurosawa Y, Fujita K, Nagatsu T (1988). Structure of the human tyrosine hydroxylase gene: alternative splicing from a single gene accounts for generation of four mRNA types. J Biochem, 103(6): 907–912
Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Hata T, Watanabe Y, Fujita K, Nagatsu T (1995). Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem, 270(45): 27235–27243
Kobayashi K, Noda Y, Matsushita N, Nishii K, Sawada H, Nagatsu T, Nakahara D, Fukabori R, Yasoshima Y, Yamamoto T, Miura M, Kano M, Mamiya T, Miyamoto Y, Nabeshima T (2000). Modest neuropsychological deficits caused by reduced noradrenaline metabolism in mice heterozygous for a mutated tyrosine hydroxylase gene. J Neurosci, 20(6): 2418–2426
Kohwi M, Osumi N, Rubenstein J L, Alvarez-Buylla A (2005). Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci, 25(30): 6997–7003
Lenartowski R, Goc A (2011). Epigenetic, transcriptional and posttranscriptional regulation of the tyrosine hydroxylase gene. Int J Dev Neurosci, 29(8): 873–883
Lewis-Tuffin L J, Quinn P G, Chikaraishi D M (2004). Tyrosine hydroxylase transcription depends primarily on cAMP response element activity, regardless of the type of inducing stimulus. Mol Cell Neurosci, 25(3): 536–547
Liu J, Merlie J P, Todd R D, O’Malley K L (1997). Identification of cell type-specific promoter elements associated with the rat tyrosine hydroxylase gene using transgenic founder analysis. Brain Res Mol Brain Res, 50(1–2): 33–42
Liu N, Cigola E, Tinti C, Jin B K, Conti B, Volpe B T, Baker H (1999). Unique regulation of immediate early gene and tyrosine hydroxylase expression in the odor-deprived mouse olfactory bulb. J Biol Chem, 274(5): 3042–3047
Lo L, Morin X, Brunet J F, Anderson D J (1999). Specification of neurotransmitter identity by Phox2 proteins in neural crest stem cells. Neuron, 22(4): 693–705
Min N, Joh T H, Kim K S, Peng C, Son J H (1994). 5′ upstream DNA sequence of the rat tyrosine hydroxylase gene directs high-level and tissue-specific expression to catecholaminergic neurons in the central nervous system of transgenic mice. Brain Res Mol Brain Res, 27(2): 281–289
Moll J R, Acharya A, Gal J, Mir A A, Vinson C (2002). Magnesium is required for specific DNA binding of the CREB B-ZIP domain. Nucleic Acids Res, 30(5): 1240–1246
Nagamoto-Combs K, Piech K M, Best J A, Sun B, Tank A W (1997). Tyrosine hydroxylase gene promoter activity is regulated by both cyclic AMP-responsive element and AP1 sites following calcium influx. Evidence for cyclic amp-responsive element binding proteinindependent regulation. J Biol Chem, 272(9): 6051–6058
Nakashima A, Ota A, Sabban E L (2003). Interactions between Egr1 and AP1 factors in regulation of tyrosine hydroxylase transcription. Brain Res Mol Brain Res, 112(1–2): 61–69
Nunes I, Tovmasian L T, Silva RM, Burke R E, Goff S P (2003). Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA, 100(7): 4245–4250
Papanikolaou N A, Sabban E L (1999). Sp1/Egr1 motif: a new candidate in the regulation of rat tyrosine hydroxylase gene transcription by immobilization stress. J Neurochem, 73(1): 433–436
Papanikolaou N A, Sabban E L (2000). Ability of Egr1 to activate tyrosine hydroxylase transcription in PC12 cells. Cross-talk with AP-1 factors. J Biol Chem, 275(35): 26683–26689
Paskin T R, Iqbal T R, Byrd-Jacobs C A (2011). Olfactory bulb recovery following reversible deafferentation with repeated detergent application in the adult zebrafish. Neuroscience, 196: 276–284
Patankar S, Lazaroff M, Yoon S O, Chikaraishi D M (1997). A novel basal promoter element is required for expression of the rat tyrosine hydroxylase gene. J Neurosci, 17(11): 4076–4086
Patel P, Nankova B B, LaGamma E F (2005). Butyrate, a gut-derived environmental signal, regulates tyrosine hydroxylase gene expression via a novel promoter element. Brain Res Dev Brain Res, 160(1): 53–62
Qin Y, Hurley L H (2008). Structures, folding patterns, and functions of intramolecular DNA G-quadruplexes found in eukaryotic promoter regions. Biochimie, 90(8): 1149–1171
Rao F, Zhang L, Wessel J, Zhang K, Wen G, Kennedy B P, Rana B K, Das M, Rodriguez-Flores J L, Smith D W, Cadman P E, Salem R M, Mahata S K, Schork N J, Taupenot L, Ziegler M G, O’Connor D T (2007). Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation, 116(9): 993–1006
Sabban E L (1997). Control of tyrosine hydroxylase gene expression in chromaffin and PC12 cells. Semin Cell Dev Biol, 8(2): 101–111
Saucedo-Cardenas O, Quintana-Hau J D, Le W D, Smidt M P, Cox J J, De Mayo F, Burbach J P, Conneely O M (1998). Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA, 95(7): 4013–4018
Schimmel J J, Crews L, Roffler-Tarlov S, Chikaraishi DM (1999). 4.5 kb of the rat tyrosine hydroxylase 5′ flanking sequence directs tissue specific expression during development and contains consensus sites for multiple transcription factors. Brain Res Mol Brain Res, 74(1–2): 1–14
Smeets W J, González A (2000). Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Brain Res Rev, 33(2–3): 308–379
Smidt M P, Smits S M, Bouwmeester H, Hamers F P, van der Linden A J, Hellemons A J, Graw J, Burbach J P (2004). Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development, 131(5): 1145–1155
Son J H, Chun H S, Joh T H, Cho S, Conti B, Lee J W (1999). Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J Neurosci, 19(1): 10–20
Stefano L, Al Sarraj J, Rössler O G, Vinson C, Thiel G (2006). Upregulation of tyrosine hydroxylase gene transcription by tetradecanoylphorbol acetate is mediated by the transcription factors Ets-like protein-1 (Elk-1) and Egr-1. J Neurochem, 97(1): 92–104
Suzuki T, Yamakuni T, Hagiwara M, Ichinose H (2002). Identification of ATF-2 as a transcriptional regulator for the tyrosine hydroxylase gene. J Biol Chem, 277(43): 40768–40774
Tan X, Zhang L, Zhu H, Qin J, Tian M, Dong C, Li H, Jin G (2014). Brn4 and TH synergistically promote the differentiation of neural stem cells into dopaminergic neurons. Neurosci Lett, 571: 23–28
Trocmé C, Sarkis C, Hermel J M, Duchateau R, Harrison S, Simonneau M, Al-Shawi R, Mallet J (1998). CRE and TRE sequences of the rat tyrosine hydroxylase promoter are required for TH basal expression in adult mice but not in the embryo. Eur J Neurosci, 10(2): 508–521
Venters B J, Pugh B F (2013). Genomic organization of human transcription initiation complexes. Nature, 502(7469): 53–58
Verbeek M M, Steenbergen-Spanjers G C, Willemsen M A, Hol F A, Smeitink J, Seeger J, Grattan-Smith P, Ryan M M, Hoffmann G F, Donati M A, Blau N, Wevers R A (2007). Mutations in the cyclic adenosine monophosphate response element of the tyrosine hydroxylase gene. Ann Neurol, 62(4): 422–426
Willemsen M A, Verbeek M M, Kamsteeg E J, de Rijk-van Andel J F, Aeby A, Blau N, Burlina A, Donati M A, Geurtz B, Grattan-Smith P J, Haeussler M, Hoffmann G F, Jung H, de Klerk J B, van der Knaap M S, Kok F, Leuzzi V, de Lonlay P, Megarbane A, Monaghan H, Renier W O, Rondot P, Ryan M M, Seeger J, Smeitink J A, Steenbergen-Spanjers G C, Wassmer E, Weschke B, Wijburg F A, Wilcken B, Zafeiriou D I, Wevers R A (2010). Tyrosine hydroxylase deficiency: a treatable disorder of brain catecholamine biosynthesis. Brain, 133(Pt 6): 1810–1822
Yamamoto K, Ruuskanen J O, Wullimann M F, Vernier P (2010). Two tyrosine hydroxylase genes in vertebrates new dopaminergic territories revealed in the zebrafish brain. Mol Cell Neurosci, 43(4): 394–402
Yamamoto K, Vernier P (2011). The evolution of dopamine systems in chordates. Front Neuroanat, 5: 21
Yamanoue Y, Miya M, Inoue J G, Matsuura K, Nishida M (2006). The mitochondrial genome of spotted green pufferfish Tetraodon nigroviridis (Teleostei: Tetraodontiformes) and divergence time estimation among model organisms in fishes. Genes Genet Syst, 81(1): 29–39
Yang C, Kim H S, Seo H, Kim K S (1998). Identification and characterization of potential cis-regulatory elements governing transcriptional activation of the rat tyrosine hydroxylase gene. J Neurochem, 71(4): 1358–1368
Yoon S O, Chikaraishi D M (1992). Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron, 9(1): 55–67
Yukimasa N, Isobe K, Nagai H, Takuwa Y, Nakai T (1999). Successive occupancy by immediate early transcriptional factors of the tyrosine hydroxylase gene TRE and CRE sites in PACAP-stimulated PC12 pheochromocytoma cells. Neuropeptides, 33(6): 475–482
Zetterström R H, Solomin L, Jansson L, Hoffer B J, Olson L, Perlmann T (1997). Dopamine neuron agenesis in Nurr1-deficient mice. Science, 276(5310): 248–250
Zhou Q Y, Quaife C J, Palmiter R D (1995). Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature, 374(6523): 640–643
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Banerjee, K., Baker, H. et al. Nucleotide sequence conservation of novel and established cis-regulatory sites within the tyrosine hydroxylase gene promoter. Front. Biol. 10, 74–90 (2015). https://doi.org/10.1007/s11515-014-1341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-014-1341-z