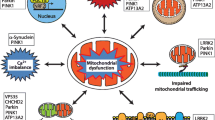
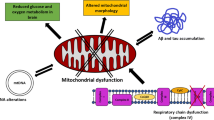
Abstract
Mitochondria are dynamic organelles which are required for maintaining cellular homeostasis. Thus, it is not surprising that irregularities in mitochondrial function result in cellular damage and are linked with neurodegenerative diseases, such as Parkinson’s disease. Evidence that mitochondrial dysfunction is key to the pathogenesis of Parkinson’s disease is founded in studies in post-mortem tissue from patients with Parkinson’s disease, and also from genetic studies stemming from patients with familial Parkinson’s disease. Whether triggered by environmental or genetic factors, mitochondrial dysfunction occurs early in the pathogenic process, and is central to Parkinson’s disease pathology. As such, targeting the mitochondria to slow or halt disease progression is an attractive strategy for disease-modifying agents in Parkinson’s disease. Indeed, several therapies which target the mitochondria have been investigated as neuroprotective treatments for Parkinson’s disease. This review will discuss the evidence supporting mitochondrial dysfunction in Parkinson’s disease pathology as well as treatment strategies that target the mitochondria.
Similar content being viewed by others
References
Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G (2009). The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem, 110(5): 1445–1456
Andres R H, Huber A W, Schlattner U, Pérez-Bouza A, Krebs S H, Seiler R W, Wallimann T, Widmer H R (2005). Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience, 133(3): 701–713
Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco T M, Thomas B, Ko H S, Sasaki M, Ischiropoulos H, Przedborski S, Dawson T M, Dawson V L (2007). DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA, 104(37): 14807–14812
Bedford L, Hay D, Devoy A, Paine S, Powe D G, Seth R, Gray T, Topham I, Fone K, Rezvani N, Mee M, Soane T, Layfield R, Sheppard P W, Ebendal T, Usoskin D, Lowe J, Mayer R J (2008). Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci, 28: 8189–8198
Beher D, Wu J, Cumine S, Kim K W, Lu S C, Atangan L, Wang M (2009). Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des, 74(6): 619–624
Bender A, Krishnan K J, Morris C M, Taylor G A, Reeve A K, Perry R H, Jaros E, Hersheson J S, Betts J, Klopstock T, Taylor R W, Turnbull DM (2006). High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet, 38(5): 515–517
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973). Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci, 20(4): 415–455
Blackinton J, Lakshminarasimhan M, Thomas K J, Ahmad R, Greggio E, Raza A S, Cookson M R, Wilson M A (2009). Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem, 284(10): 6476–6485
Bové J, Zhou C, Jackson-Lewis V, Taylor J, Chu Y, Rideout H J, Wu D C, Kordower J H, Petrucelli L, Przedborski S (2006). Proteasome inhibition and Parkinson’s disease modeling. Ann Neurol, 60(2): 260–264
Brunet A, Sweeney L B, Sturgill J F, Chua K F, Greer P L, Lin Y, Tran H, Ross S E, Mostoslavsky R, Cohen H Y, Hu L S, Cheng H L, Jedrychowski M P, Gygi S P, Sinclair D A, Alt F W, Greenberg M E (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science, 303(5666): 2011–2015
Canet-Avilés R M, Wilson M A, Miller D W, Ahmad R, McLendon C, Bandyopadhyay S, Baptista M J, Ringe D, Petsko G A, Cookson MR (2004). The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA, 101(24): 9103–9108
Chan C S, Gertler T S, Surmeier D J (2010). A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord, 25(Suppl 1): S63–70
Chan C S, Guzman J N, Ilijic E, Mercer J N, Rick C, Tkatch T, Meredith G E, Surmeier D J (2007). ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature, 447(7148): 1081–1086
Chao J, Yu M S, Ho Y S, Wang M, Chang R C (2008). Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med, 45(7): 1019–1026
Chartier-Harlin M C, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A (2004). Alphasynuclein locus duplication as a cause of familial Parkinson’s disease. Lancet, 364(9440): 1167–1169
Cherra S J 3rd, Steer E, Gusdon A M, Kiselyov K, Chu C T (2013). Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am J Pathol, 182(2): 474–484
Chinta S J, Mallajosyula J K, Rane A, Andersen J K (2010). Mitochondrial α-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett, 486(3): 235–239
Ciron C, Lengacher S, Dusonchet J, Aebischer P, Schneider B L (2012). Sustained expression of PGC-1α in the rat nigrostriatal system selectively impairs dopaminergic function. Hum Mol Genet, 21(8): 1861–1876
Clark I E, Dodson M W, Jiang C, Cao J H, Huh J R, Seol J H, Yoo S J, Hay B A, Guo M (2006). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature, 441(7097): 1162–1166
Cole N B, Murphy D D (2002). The cell biology of alpha-synuclein: a sticky problem? Neuromolecular Med, 1(2): 95–109
Cookson M R (2003). Parkin’s substrates and the pathways leading to neuronal damage. Neuromolecular Med, 3(1): 1–13
Couzin J (2007). Clinical research. Testing a novel strategy against Parkinson’s disease. Science, 315(5820): 1778
Cuervo A M, Stefanis L, Fredenburg R, Lansbury P T, Sulzer D (2004). Impaired degradation of mutant alpha-synuclein by chaperonemediated autophagy. Science, 305(5688): 1292–1295
Dauer W, Przedborski S (2003). Parkinson’s disease: mechanisms and models. Neuron, 39(6): 889–909
Devi L, Raghavendran V, Prabhu B M, Avadhani N G, Anandatheerthavarada H K (2008). Mitochondrial import and accumulation of alphasynuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem, 283(14): 9089–9100
Donmez G, Arun A, Chung C Y, McLean P J, Lindquist S, Guarente L (2012). SIRT1 protects against alpha-synuclein aggregation by activating molecular chaperones. J Neurosci, 32: 124–132
Dorsey E R, Constantinescu R, Thompson J P, Biglan K M, Holloway R G, Kieburtz K, Marshall F J, Ravina B M, Schifitto G, Siderowf A, Tanner C M (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology, 68(5): 384–386
Dryanovski D I, Guzman J N, Xie Z, Galteri D J, Volpicelli-Daley L A, Lee V M, Miller R J, Schumacker P T, Surmeier D J (2013). Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci, 33(24): 10154–10164
Ekstrand M I, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson F S, Trifunovic A, Hoffer B, Cullheim S, Mohammed A H, Olson L, Larsson N G (2007). Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci USA, 104(4): 1325–1330
Esteves A R, Lu J, Rodova M, Onyango I, Lezi E, Dubinsky R, Lyons K E, Pahwa R, Burns J M, Cardoso S M, Swerdlow R H (2010). Mitochondrial respiration and respiration-associated proteins in cell lines created through Parkinson’s subject mitochondrial transfer. J Neurochem, 113(3): 674–682
Exner N, Lutz A K, Haass C, Winklhofer K F (2012). Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J, 31(14): 3038–3062
Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell’aquila C, De Mari M, Ferranini E, Bonifati V, Pacelli C, Cocco T (2014). Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson’s disease. Biochim Biophys Acta, 1842(7): 902–915
Frye R A (2000). Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun, 273(2): 793–798
Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson E M, Schüle B, Langston J W, Middleton F A, Ross O A, Hulihan M, Gasser T, Farrer M J (2007). Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology, 68(12): 916–922
Gautier C A, Kitada T, Shen J (2008). Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA, 105(32): 11364–11369
Gerhart-Hines Z, Rodgers J T, Bare O, Lerin C, Kim S H, Mostoslavsky R, Alt F W, Wu Z, Puigserver P (2007). Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J, 26(7): 1913–1923
Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken H H, Becker D, Voos W, Leuner K, Müller W E, Kudin A P, Kunz W S, Zimmermann A, Roeper J, Wenzel D, Jendrach M, García-Arencíbia M, Fernández-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert A S, Rüb U, Chen A, Nussbaum R L, Auburger G (2009). Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS ONE, 4(6): e5777
Goedert M (2001). Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci, 2(7): 492–501
Goldberg M S, Fleming S M, Palacino J J, Cepeda C, Lam H A, Bhatnagar A, Meloni E G, Wu N, Ackerson L C, Klapstein G J, Gajendiran M, Roth B L, Chesselet M F, Maidment N T, Levine M S, Shen J (2003). Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem, 278(44): 43628–43635
Gómez-Sánchez R, Gegg M E, Bravo-San Pedro J M, Niso-Santano M, Alvarez-Erviti L, Pizarro-Estrella E, Gutiérrez-Martín Y, Alvarez-Barrientos A, Fuentes JM, González-Polo R A, Schapira A H (2014). Mitochondrial impairment increases FL-PINK1 levels by calciumdependent gene expression. Neurobiol Dis, 62: 426–440
González-Polo R, Niso-Santano M, Morán JM, Ortiz-Ortiz MA, Bravo-San Pedro J M, Soler G, Fuentes J M (2009). Silencing DJ-1 reveals its contribution in paraquat-induced autophagy. J Neurochem, 109(3): 889–898
Good C H, Hoffman A F, Hoffer B J, Chefer V I, Shippenberg T S, Backman CM, Larsson N G, Olson L, Gellhaar S, Galter D, Lupica C R (2011). Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson’s disease. FASEB J, 25:1333–1344
Greenamyre J T, Betarbet R, Sherer T B (2003). The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism Relat Disord, 9(Suppl 2): S59–S64
Greene J C, Whitworth A J, Kuo I, Andrews L A, Feany M B, Pallanck L J (2003). Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA, 100(7): 4078–4083
Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug M P, Beilina A, Blackinton J, Thomas K J, Ahmad R, Miller D W, Kesavapany S, Singleton A, Lees A, Harvey R J, Harvey K, Cookson M R (2006). Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis, 23(2): 329–341
Gu G, Reyes P E, Golden G T, Woltjer R L, Hulette C, Montine T J, Zhang J (2002). Mitochondrial DNA deletions/rearrangements in parkinson disease and related neurodegenerative disorders. J Neuropathol Exp Neurol, 61(7): 634–639
Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon E A, Przedborski S (2014). alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosc, 34: 249–259
Guzman J N, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker P T, Surmeier D J (2010). Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature, 468(7324): 696–700
Haas R H, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults C W (1995). Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann Neurol, 37(6): 714–722
Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, Kanki T, Kang D, Sunagawa K, Tsutsui H, Nakanishi H (2008). Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci, 28: 8624–8634
Healy D G, Abou-Sleiman P M, Casas J P, Ahmadi K R, Lynch T, Gandhi S, Muqit M M, Foltynie T, Barker R, Bhatia K P, Quinn N P, Lees A J, Gibson J M, Holton J L, Revesz T, Goldstein D B, Wood N W (2006). UCHL-1 is not a Parkinson’s disease susceptibility gene. Ann Neurol, 59(4): 627–633
Höglinger G U, Carrard G, Michel P P, Medja F, Lombès A, Ruberg M, Friguet B, Hirsch E C (2003). Dysfunction of mitochondrial complex I and the proteasome: interactions between two biochemical deficits in a cellular model of Parkinson’s disease. J Neurochem, 86(5): 1297–1307
Howitz K T, Bitterman K J, Cohen H Y, Lamming DW, Lavu S, Wood J G, Zipkin R E, Chung P, Kisielewski A, Zhang L L, Scherer B, Sinclair D A (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature, 425(6954): 191–196
Ikebe S, Tanaka M, Ozawa T (1995). Point mutations of mitochondrial genome in Parkinson’s disease. Brain Res Mol Brain Res, 28(2): 281–295
Imai Y, Soda M, Takahashi R (2000). Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem, 275(46): 35661–35664
Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, Yanagisawa D, Nishimura K, Taniguchi T, Kiso Y, Yoshimoto K, Agatsuma T, Koide-Yoshida S, Iguchi-Ariga S M, Shimohama S, Ariga H (2006). PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol Dis, 24(1): 144–158
Irrcher I, Aleyasin H, Seifert E L, Hewitt S J, Chhabra S, Phillips M, Lutz A K, Rousseaux M W, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin J G, Winklhofer K F, Rizzu P, Rippstein P, Kim R H, Chen C X, Fon E A, Slack R S, Harper M E, McBride H M, Mak T W, Park D S (2010). Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet, 19(19): 3734–3746
Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme G A, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos M J, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney T A, Brice A, Garcia de Yebenes J (2003). Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet, 12(18): 2277–2291
Jin F, Wu Q, Lu Y F, Gong Q H, Shi J S (2008). Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol, 600(1-3): 78–82
Juhn M S, Tarnopolsky M (1998). Oral creatine supplementation and athletic performance: a critical review. Clin J Sport Med, 8: 286–297
Kakefuda K, Fujita Y, Oyagi A, Hyakkoku K, Kojima T, Umemura K, Tsuruma K, Shimazawa M, Ito M, Nozawa Y, Hara H (2009). Sirtuin 1 overexpression mice show a reference memory deficit, but not neuroprotection. Biochem Biophys Res Commun, 387(4): 784–788
Katzenschlager R, Lees A J (2002). Treatment of Parkinson’s disease: levodopa as the first choice. J Neurol, 249(Suppl 2): II19–II24
Keeney P M, Quigley C K, Dunham L D, Papageorge C M, Iyer S, Thomas R R, Schwarz K M, Trimmer P A, Khan S M, Portell F R, Bergquist K E, Bennett J P Jr (2009). Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson’s disease cell model. Hum Gene Ther, 20(8): 897–907
Kim R H, Smith P D, Aleyasin H, Hayley S, Mount M P, Pownall S, Wakeham A, You-Ten A J, Kalia S K, Horne P, Westaway D, Lozano A M, Anisman H, Park D S, Mak T W (2005). Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA, 102(14): 5215–5220
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature, 392(6676): 605–608
Kitada T, Pisani A, Porter D R, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos E N, Shen J (2007). Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA, 104(27): 11441–11446
Klivenyi P, Gardian G, Calingasan N Y, Yang L, Beal M F (2003). Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Mol Neurosci, MN 21: 191–198
Klivenyi P, Calingasan N Y, Starkov A, Stavrovskaya I G, Kristal B S, Yang L, Wieringa B, Beal M F (2004). Neuroprotective mechanisms of creatine occur in the absence of mitochondrial creatine kinase. Neurobiol Dis, 15(3): 610–617
Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante R J, Kowall N W, Abeliovich A, Beal M F (2006). Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis, 21(3): 541–548
Kones R (2010). Mitochondrial therapy for Parkinson’s disease: neuroprotective pharmaconutrition may be disease-modifying. Clin pharmacol, 2: 185–198
Kordower J H, Kanaan N M, Chu Y, Suresh Babu R, Stansell J 3rd, Terpstra B T, Sortwell C E, Steece-Collier K, Collier T J (2006). Failure of proteasome inhibitor administration to provide a model of Parkinson’s disease in rats and monkeys. Ann Neurol, 60(2): 264–268
Kraytsberg Y, Kudryavtseva E, McKee A C, Geula C, Kowall N W, Khrapko K (2006). Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet, 38(5): 518–520
Krebiehl G, Ruckerbauer S, Burbulla L F, Kieper N, Maurer B, Waak J, Wolburg H, Gizatullina Z, Gellerich F N, Woitalla D, Riess O, Kahle P J, Proikas-Cezanne T, Krüger R (2010). Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS ONE, 5(2): e9367
Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen J T, Schöls L, Riess O (1998). Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet, 18(2): 106–108
Lakshminarasimhan M, Rauh D, Schutkowski M, Steegborn C (2013). Sirt1 activation by resveratrol is substrate sequence-selective. Aging (Albany NY), 5(3): 151–154
Langston J W, Ballard P, Tetrud J W, Irwin I (1983). Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science, 219(4587): 979–980
Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein M J, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach P J, Wilkinson K D, Polymeropoulos M H (1998). The ubiquitin pathway in Parkinson’s disease. Nature, 395(6701): 451–452
Li C, Beal M F (2005). Leucine-rich repeat kinase 2: a new player with a familiar theme for Parkinson’s disease pathogenesis. Proc Natl Acad Sci USA, 102(46): 16535–16536
Li X, Kazgan N (2011). Mammalian sirtuins and energy metabolism. Int J Biol Sci, 7(5): 575–587
Lim K L (2007). Ubiquitin-proteasome system dysfunction in Parkinson’s disease: current evidence and controversies. Expert Rev Proteomics, 4(6): 769–781
Lin T K, Chen S D, Chuang Y C, Lin H Y, Huang C R, Chuang J H, Wang P W, Huang S T, Tiao M M, Chen J B, Liou C W (2014). Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int J Mol Sci, 15(1): 1625–1646
Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson M P (2009). Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med, 11(1): 28–42
Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Uéda K, Chan P, Yu S (2009). alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett, 454(3): 187–192
Lu K T, Ko M C, Chen B Y, Huang J C, Hsieh C W, Lee M C, Chiou R Y, Wung B S, Peng C H, Yang Y L (2008). Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem, 56(16): 6910–6913
Lutz A K, Exner N, Fett M E, Schlehe J S, Kloos K, Lämmermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert A S, Bouman L, Vogt-Weisenhorn D, Wurst W, Tatzelt J, Haass C, Winklhofer K F (2009). Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem, 284(34): 22938–22951
Marques O, Outeiro T F (2012). Alpha-synuclein: from secretion to dysfunction and death. Cell Death Dis, 3(7): e350
Matthews R T, Ferrante R J, Klivenyi P, Yang L, Klein A M, Mueller G, Kaddurah-Daouk R, Beal M F (1999). Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol, 157(1): 142–149
McLelland G L, Soubannier V, Chen C X, McBride H M, Fon E A (2014). Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J, 33(4): 282–295
McNaught K S, Perl D P, Brownell A L, Olanow C W (2004). Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol, 56(1): 149–162
Minakawa E N, Yamakado H, Tanaka A, Uemura K, Takeda S, Takahashi R (2013). Chicken DT40 cell line lacking DJ-1, the gene responsible for familial Parkinson’s disease, displays mitochondrial dysfunction. Neurosci Res, 77(4): 228–233
Moisoi N, Fedele V, Edwards J, Martins L M (2014). Loss of PINK1 enhances neurodegeneration in a mouse model of Parkinson’s disease triggered by mitochondrial stress. Neuropharmacology, 77: 350–357
Morais V A, Haddad D, Craessaerts K, De Bock P J, Swerts J, Vilain S, Aerts L, Overbergh L, Grünewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B (2014). PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science, 344(6180): 203–207
Mortiboys H, Johansen K K, Aasly J O, Bandmann O (2010). Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology, 75(22): 2017–2020
Mortiboys H, Thomas K J, Koopman W J, Klaffke S, Abou-Sleiman P, Olpin S, Wood N W, Willems P H, Smeitink J A, Cookson M R, Bandmann O (2008). Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol, 64(5): 555–565
Mudò G, Mäkelä J, Di Liberto V, Tselykh T V, Olivieri M, Piepponen P, Eriksson O, Mälkiä A, Bonomo A, Kairisalo M, Aguirre J A, Korhonen L, Belluardo N, Lindholm D (2012). Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci, 69(7): 1153–1165
Murray A M, Weihmueller F B, Marshall J F, Hurtig H I, Gottleib G L, Joyce J N (1995). Damage to dopamine systems differs between Parkinson’s disease and Alzheimer’s disease with parkinsonism. Ann Neurol, 37(3): 300–312
Narendra D, Tanaka A, Suen D F, Youle R J (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol, 183(5): 795–803
Neuspiel M, Schauss A C, Braschi E, Zunino R, Rippstein P, Rachubinski R A, Andrade-Navarro M A, McBride H M (2008). Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol, CB 18: 102–108
Ng C H, Mok S Z, Koh C, Ouyang X, Fivaz M L, Tan E K, Dawson V L, Dawson T M, Yu F, Lim K L (2009). Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci, 29: 11257–11262
NINDS NET-PD Investigators (2006). A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology, 66(5): 664–671
Nishiyama S, Shitara H, Nakada K, Ono T, Sato A, Suzuki H, Ogawa T, Masaki H, Hayashi J, Yonekawa H (2010). Over-expression of Tfam improves the mitochondrial disease phenotypes in a mouse model system. Biochem Biophys Res Commun, 401(1): 26–31
Niu J, Yu M, Wang C, Xu Z (2012). Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein. J Neurochem, 122(3): 650–658
Noack H, Bednarek T, Heidler J, Ladig R, Holtz J, Szibor M (2006). TFAM-dependent and independent dynamics of mtDNA levels in C2C12 myoblasts caused by redox stress. Biochim Biophys Acta, 1760(2): 141–150
O’Donnell K C, Lulla A, Stahl M C, Wheat N D, Bronstein J M, Sagasti A (2014). Axon degeneration and PGC-1α-mediated protection in a zebrafish model of α-synuclein toxicity. Dis Model Mech, 7(5): 571–582
Orenstein S J, Kuo S H, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig L S, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo A M (2013). Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci, 16(4): 394–406
Pacholec M, Bleasdale J E, Chrunyk B, Cunningham D, Flynn D, Garofalo R S, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K (2010). SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem, 285(11): 8340–8351
Papkovskaia T D, Chau K Y, Inesta-Vaquera F, Papkovsky D B, Healy D G, Nishio K, Staddon J, Duchen M R, Hardy J, Schapira A H, Cooper J M (2012). G2019S leucine-rich repeat kinase 2 causes uncoupling protein-mediated mitochondrial depolarization. Hum Mol Genet, 21(19): 4201–4213
Pardo P S, Mohamed J S, Lopez M A, Boriek A M (2011). Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J Biol Chem, 286(4): 2559–2566
Parihar M S, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008). Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci, 65(7-8): 1272–1284
Park J, Kim S Y, Cha G H, Lee S B, Kim S, Chung J (2005). Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene, 361: 133–139
Park J, Lee S B, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim J M, Chung J (2006). Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature, 441(7097): 1157–1161
Parker W D Jr, Boyson S J, Parks J K (1989). Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol, 26(6): 719–723
Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G (2004). Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development, 131(9): 2183–2194
Ping H X, Shepard P D (1999). Blockade of SK-type Ca2+-activated K+ channels uncovers a Ca2+-dependent slow afterdepolarization in nigral dopamine neurons. J Neurophysiol, 81(3): 977–984
Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos E S, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson W G, Lazzarini A M, Duvoisin R C, Di Iorio G, Golbe L I, Nussbaum R L (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science, 276(5321): 2045–2047
Poole A C, Thomas R E, Andrews L A, McBride H M, Whitworth A J, Pallanck L J (2008). The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA, 105(5): 1638–1643
Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid L P, Goebel I, Mubaidin A F, Wriekat A L, Roeper J, Al-Din A, Hillmer A M, Karsak M, Liss B, Woods C G, Behrens M I, Kubisch C (2006). Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet, 38(10): 1184–1191
Ramonet D, Daher J P, Lin BM, Stafa K, Kim J, Banerjee R, Westerlund M, Pletnikova O, Glauser L, Yang L, Liu Y, Swing D A, Beal M F, Troncoso J C, McCaffery JM, Jenkins N A, Copeland N G, Galter D, Thomas B, Lee M K, Dawson T M, Dawson V L, Moore D J (2011). Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS ONE, 6(4): e18568
Richfield E K, Thiruchelvam M J, Cory-Slechta D A, Wuertzer C, Gainetdinov R R, Caron M G, Di Monte D A, Federoff H J (2002). Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol, 175(1): 35–48
Saha S, Guillily M D, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu C H, Segal L, Raghavan K, Matsumoto K, Hisamoto N, Kuwahara T, Iwatsubo T, Moore L, Goldstein L, Cookson M, Wolozin B (2009). LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci, 29: 9210–9218
Sakata E, Yamaguchi Y, Kurimoto E, Kikuchi J, Yokoyama S, Yamada S, Kawahara H, Yokosawa H, Hattori N, Mizuno Y, Tanaka K, Kato K (2003). Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep, 4(3): 301–306
Sarafian T A, Ryan C M, Souda P, Masliah E, Kar U K, Vinters H V, Mathern G W, Faull K F, Whitelegge J P, Watson J B (2013). Impairment of mitochondria in adult mouse brain overexpressing predominantly full-length, N-terminally acetylated human α-synuclein. PLoS ONE, 8(5): e63557 PMID:23667637
Scarffe L A, Stevens D A, Dawson V L, Dawson T M (2014). Parkin and PINK1: much more than mitophagy. Trends Neurosci, 37(6): 315–324
Schapira A H, Cooper J M, Dexter D, Jenner P, Clark J B, Marsden C D (1989). Mitochondrial complex I deficiency in Parkinson’s disease. Lancet, 1(8649): 1269
Shavali S, Brown-Borg H M, Ebadi M, Porter J (2008). Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett, 439(2): 125–128
Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T (2000). Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet, 25(3): 302–305
Shin J H, Ko H S, Kang H, Lee Y, Lee Y I, Pletinkova O, Troconso J C, Dawson V L, Dawson T M (2011). PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell, 144(5): 689–702
Simon D K, Lin M T, Zheng L, Liu G J, Ahn C H, Kim L M, Mauck W M, Twu F, Beal M F, Johns D R (2004). Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson’s disease. Neurobiol Aging, 25(1): 71–81
Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B (2003). Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem, 278(14): 11753–11759
Song D D, Shults C W, Sisk A, Rockenstein E, Masliah E (2004). Enhanced substantia nigra mitochondrial pathology in human alphasynuclein transgenic mice after treatment with MPTP. Exp Neurol, 186(2): 158–172
Soubannier V, McLelland G L, Zunino R, Braschi E, Rippstein P, Fon E A, McBride H M (2012). A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol, CB 22: 135–141
Spillantini M G, Crowther R A, Jakes R, Hasegawa M, Goedert M (1998). alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA, 95(11): 6469–6473
Spillantini M G, Schmidt M L, Lee V M, Trojanowski J Q, Jakes R, Goedert M (1997). Alpha-synuclein in Lewy bodies. Nature, 388(6645): 839–840
St-Pierre J, Drori S, Uldry M, Silvaggi J M, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon D K, Bachoo R, Spiegelman B M (2006). Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell, 127(2): 397–408
Su Y C, Qi X (2013). Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum Mol Genet, 22(22): 4545–4561
Sulzer D, Zecca L (2000). Intraneuronal dopamine-quinone synthesis: a review. Neurotox Res, 1(3): 181–195
Surmeier D J (2007). Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol, 6(10): 933–938
Surmeier D J, Guzman J N, Sanchez-Padilla J (2010). Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease. Cell Calcium, 47(2): 175–182
Taira T, Saito Y, Niki T, Iguchi-Ariga S M, Takahashi K, Ariga H (2004). DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep, 5(2): 213–218
Tanaka M, Kim Y M, Lee G, Junn E, Iwatsubo T, Mouradian M M (2004). Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem, 279(6): 4625–4631
Valente E M, Abou-Sleiman P M, Caputo V, Muqit M M, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio A R, Healy D G, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks W P, Latchman D S, Harvey R J, Dallapiccola B, Auburger G, Wood N W (2004). Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science, 304(5674): 1158–1160
Valente E M, Salvi S, Ialongo T, Marongiu R, Elia A E, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio A R (2004). PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol, 56(3): 336–341
van der Horst A, Tertoolen L G, de Vries-Smits L M, Frye R A, Medema R H, Burgering B M (2004). FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1). J Biol Chem, 279(28): 28873–28879
Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D (2007). SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature, 450(7168): 440–444
Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D (2004). Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell, 16(1): 93–105
Wang X, Winter D, Ashrafi G, Schlehe J, Wong Y L, Selkoe D, Rice S, Steen J, LaVoie M J, Schwarz T L (2011). PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell, 147(4): 893–906
Wang X, Yan M H, Fujioka H, Liu J, Wilson-Delfosse A, Chen S G, Perry G, Casadesus G, Zhu X (2012). LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet, 21(9): 1931–1944
Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A (2009). PGC-1alpha and PGC-1beta regulate mitochondrial density in neurons. J Biol Chem, 284(32): 21379–21385
West A B, Moore D J, Biskup S, Bugayenko A, Smith W W, Ross C A, Dawson V L, Dawson T M (2005). Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA, 102(46): 16842–16847
Westerheide S D, Anckar J, Stevens S M Jr, Sistonen L, Morimoto R I (2009). Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science, 323(5917): 1063–1066
Winslow A R, Chen C W, Corrochano S, Acevedo-Arozena A, Gordon D E, Peden A A, Lichtenberg M, Menzies F M, Ravikumar B, Imarisio S, Brown S, O’Kane C J, Rubinsztein D C (2010). α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol, 190(6): 1023–1037
Wood-Kaczmar A, Gandhi S, Yao Z, Abramov A Y, Miljan E A, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen M R, Latchman D, Tabrizi S J, Wood N W (2008). PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE, 3(6): e2455
Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L (2009). Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS ONE, 4(5): e5515
Yang S R, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I (2007). Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cel l Mol Physiol, 292(2): L567–L576
Yeung F, Hoberg J E, Ramsey C S, Keller M D, Jones D R, Frye R A, Mayo M W (2004). Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J, 23(12): 2369–2380
Yong-Kee C J, Salomonczyk D, Nash J E (2011). Development and validation of a screening assay for the evaluation of putative neuroprotective agents in the treatment of Parkinson’s disease. Neurotox Res, 19(4): 519–526
Yong-Kee C J, Sidorova E, Hanif A, Perera G, Nash J E (2012). Mitochondrial dysfunction precedes other sub-cellular abnormalities in an in vitro model linked with cell death in Parkinson’s disease. Neurotox Res, 21(2): 185–194
Zarranz J J, Alegre J, Gómez-Esteban J C, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz D G, de Yebenes J G (2004). The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol, 55(2): 164–173
Zhang L, Shimoji M, Thomas B, Moore D J, Yu S W, Marupudi N I, Torp R, Torgner I A, Ottersen O P, Dawson T M, Dawson V L (2005). Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet, 14(14): 2063–2073
Zhang N Y, Tang Z, Liu CW (2008). alpha-Synuclein protofibrils inhibit 26 S proteasome-mediated protein degradation: understanding the cytotoxicity of protein protofibrils in neurodegenerative disease pathogenesis. J Biol Chem, 283(29): 20288–20298
Zheng B, Liao Z, Locascio J J, Lesniak K A, Roderick S S, Watt M L, Eklund A C, Zhang-James Y, Kim P D, Hauser M A, Grünblatt E, Moran L B, Mandel S A, Riederer P, Miller R M, Federoff H J, Wüllner U, Papapetropoulos S, Youdim M B, Cantuti-Castelvetri I, Young A B, Vance J M, Davis R L, Hedreen J C, Adler C H, Beach T G, Graeber M B, Middleton F A, Rochet J C, Scherzer C R, Global P D G E C, and the Global PD Gene Expression (GPEX) Consortium (2010). PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med, 2(52): 52ra73
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gleave, J.A., Perri, P.D. & Nash, J.E. Mitochondrial dysfunction in Parkinson’s disease: a possible target for neuroprotection. Front. Biol. 9, 489–503 (2014). https://doi.org/10.1007/s11515-014-1337-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-014-1337-8