Abstract
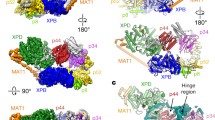
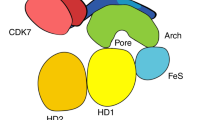
Xeroderma pigmentosum group B (XPB) and D (XPD) are two DNA helicases inside the transcription factor TFIIH complex required for both transcription and DNA repair. The importance of these helicases is underscored by the fact that mutations of XPB and XPD cause diseases with extremely high sensitivity to UV-light and high risk of cancer, premature aging, etc. This mini-review focuses on recent developments in both structural and functional characterization of these XP helicases to illustrate their distinguished biological roles within the architectural restriction of the TFIIH complex. In particular, molecular mechanisms of DNA unwinding by these helicases for promoter opening during transcription initiation and bubble-creation around the lesion during DNA repair are described based on the integration of the crystal structures of XPB and XPD helicases into the architecture of the TFIIH complex.
Similar content being viewed by others
References
Chang W H, Kornberg R D (2000). Electron crystal structure of the transcription factor and DNA repair complex, core TFIIH. Cell, 102(5): 609–613
Compe E, Egly J M (2012). TFIIH: when transcription met DNA repair. Nat Rev Mol Cell Biol, 13(6): 343–354
Egly J M, Coin F (2011). A history of TFIIH: two decades of molecular biology on a pivotal transcription/repair factor. DNA Repair (Amst), 10(7): 714–721
Fan L, Arvai A S, Cooper P K, Iwai S, Hanaoka F, Tainer J A (2006). Conserved XPB core structure and motifs for DNA unwinding: implications for pathway selection of transcription or excision repair. Mol Cell, 22(1): 27–37
Fan L, Fuss J O, Cheng Q J, Arvai A S, Hammel M, Roberts V A, Cooper P K, Tainer J A (2008). XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations. Cell, 133(5): 789–800
Fuss J O, Tainer J A (2011). XPB and XPD helicases in TFIIH orchestrate DNA duplex opening and damage verification to coordinate repair with transcription and cell cycle via CAK kinase. DNA Repair (Amst), 10(7): 697–713
Gillet L C J, Schärer O D (2006). Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev, 106(2): 253–276
Hanawalt P C, Spivak G (2008). Transcription-coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Biol, 9(12): 958–970
Hilario E, Li Y, Nobumori Y, Liu X, Fan L (2013). Structure of the Cterminal half of human XPB helicase and the impact of the diseasecausing mutation XP11BE. Acta Crystallogr D Biol Crystallogr, 69(Pt 2): 237–246
Kim T K, Ebright R H, Reinberg D (2000). Mechanism of ATPdependent promoter melting by transcription factor IIH. Science, 288(5470): 1418–1422
Kuper J, Kisker C (2013). DNA Helicases in NER, BER, and MMR. Adv Exp Med Biol, 767: 203–224
Liu H, Rudolf J, Johnson K A, McMahon S A, Oke M, Carter L, McRobbie A M, Brown S E, Naismith J H, White M F (2008). Structure of the DNA repair helicase XPD. Cell, 133(5): 801–812
Mathieu N, Kaczmarek N, Naegeli H (2010). Strand- and site-specific DNA lesion demarcation by the xeroderma pigmentosum group D helicase. Proc Natl Acad Sci U S A, 107(41): 17545–17550
Min J H, Pavletich N P (2007). Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature, 449(7162): 570–575
Naegeli H, Modrich P, Friedberg E C (1993). The DNA helicase activities of Rad3 protein of Saccharomyces cerevisiae and helicase II of Escherichia coli are differentially inhibited by covalent and noncovalent DNA modifications. J Biol Chem, 268(14): 10386–10392
Naegeli H, Sugasawa K (2011). The xeroderma pigmentosum pathway: decision tree analysis of DNA quality. DNA Repair (Amst), 10(7): 673–683
Oksenych V, Bernardes de Jesus B, Zhovmer A, Egly J M, Coin F (2009). Molecular insights into the recruitment of TFIIH to sites of DNA damage. EMBO J, 28(19): 2971–2980
Roth H M, Römer J, Grundler V, Van Houten B, Kisker C, Tessmer I (2012). XPB helicase regulates DNA incision by the Thermoplasma acidophilum endonuclease Bax1. DNA Repair (Amst), 11(3): 286–293
Rouillon C, White M F (2010). The XBP-Bax1 helicase-nuclease complex unwinds and cleaves DNA: implications for eukaryal and archaeal nucleotide excision repair. J Biol Chem, 285(14): 11013–11022
Sarker A H, Tsutakawa S E, Kostek S, Ng C, Shin D S, Peris M, Campeau E, Tainer J A, Nogales E, Cooper P K (2005). Recognition of RNA polymerase II and transcription bubbles by XPG, CSB, and TFIIH: insights for transcription-coupled repair and Cockayne Syndrome. Mol Cell, 20(2): 187–198
Schultz P, Fribourg S, Poterszman A, Mallouh V, Moras D, Egly J M (2000). Molecular structure of human TFIIH. Cell, 102(5): 599–607
Singleton M R, Dillingham M S, Wigley D B (2007). Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem, 76: 23–50
Wolski S C, Kuper J, Hänzelmann P, Truglio J J, Croteau D L, Van Houten B, Kisker C (2008). Crystal structure of the FeS clustercontaining nucleotide excision repair helicase XPD. PLoS Biol, 6(6): e149
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, L. How two helicases work together within the TFIIH complex, a perspective from structural studies of XPB and XPD helicases. Front. Biol. 8, 363–368 (2013). https://doi.org/10.1007/s11515-013-1259-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-013-1259-x