Abstract
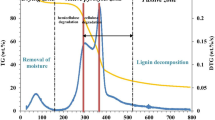
The advanced isoconversional and nonlinear model-fitting methods were used to calculate the activation energies of the thermal process of polysaccharide iron complex (PIC). The results of TG/TDG indicate that the processing temperature of the food should not exceed 223 °C when PIC is used as a food additive. The calculated results indicated the decomposition process involved two stages. The region I in stage II is a kinetically complex process, while stage I and region II of stage II are all single-step processes. The most probable mechanisms for stage I and region II of stage II are chemical reaction and contracting sphere. Meanwhile, the most probable mechanism functions for region I of stage II were determined by using nonlinear model-fitting method. The results of nonlinear model-fitting method showed the most probable mechanism of the parallel reactions for region I of stage II were nucleation and branching nuclei.





Similar content being viewed by others
Abbreviations
- a :
-
slope of equation
- α :
-
conversion degree
- α max :
-
peak conversion degree of DTG
- A :
-
pre-exponential factor (s−1)
- A 0 :
-
pre-exponential factor (s−1)
- A i :
-
pre-exponential factor (s−1)
- b :
-
intercept of equation (s−1)
- β :
-
heating rate (K/min)
- β i :
-
heating rate (K/min)
- β j :
-
heating rate (K/min)
- e :
-
Neper number
- E α :
-
apparent activation energy (kJ/mol)
- E i :
-
apparent activation energy (kJ/mol)
- E 0 :
-
apparent activation energy (kJ/mol)
- f(α):
-
differentia form of mechanism function
- G(α):
-
integral form of mechanism function
- g i (α):
-
integral form of mechanism function
- ΔG ≠ :
-
free energy of activation (kJ/mol)
- h :
-
Planck constant (6.626 × 10−34 J s)
- ΔH ≠ :
-
enthalpy of activation (kJ/mol)
- I :
-
temperature integral of thermal decomposition kinetic equation for solid-state material
- k 1 :
-
thermal decomposition rate constant (s−1)
- k 2 :
-
thermal decomposition rate constant (s−1)
- k B :
-
Boltzmann constant (J K−1)
- n:
-
reaction order
- p(x):
-
one of intermediate variable obtained by x
- Ω :
-
minimum value obtained by Vyazovkin equation
- R :
-
gas constant = 8.314 (J mol−1 K−1)
- RSS :
-
minimum value obtained by nonlinear model-fitting method equation
- ΔS≠ :
-
entropy of activation (J mol−1 K−1)
- T :
-
thermal decomposition temperature (K)
- T α :
-
thermal decomposition temperature (K)
- T max :
-
peak temperature of DTG curve (K)
- T p :
-
peak temperature of DTG curve (K)
- x :
-
one of intermediate variable obtained by E α ,R and T
- x 1 :
-
proportionality coefficient
- x 2 :
-
proportionality coefficient
- χ :
-
the transmission factor
- Y exp :
-
value of mechanism function by experiment
- Y cal :
-
value of mechanism function by g(α) equation
References
J. Zheng, X.L. Yue, Z.F. Dai, Y. Wang, S.Q. Liu, X.F. Yan, Acta Biomater. 5, 1499–1507 (2009)
E.M. Coe, L.H. Bowen, J.A. Speer, Z. Wang, D.E. Sayers, R.D. Bereman, J. Inorg, Biochem. 58(4), 269–278 (1995)
L. Piccinni, M. Ricciotti, Panminerva Med. 24(3), 213–320 (1982)
G.M. Bornhorst, R.P. Singh, Food Biophys. 8(1), 50–59 (2013)
S. Mack, M.A. Hussein, T. Becker, Food Biophys. 8(1), 69–79 (2013)
M. Jekle, T. Becker, Food Biophys. 7(3), 200–208 (2012)
I. Doymaz, Food Biophys. 6(4), 461–467 (2011)
V. Kontogiorgos, S.M. Tosh, P.J. Wood, Food Biophys. 4(3), 240–247 (2009)
S. Vyazovkin, A.K. Burnham, J.M. Criado, L.A. Pérez-Maqueda, C. Popescu, N. Sbirrazzuoli, Thermochim. Acta 520(1–2), 1–19 (2011)
S. Vyazovkin, J. Comput, Chem. 22(2), 178–183 (2001)
J.T. Wan, C. Li, H. Fan, Z.Y. Bu, B.G. Li, Thermochim. Acta 544, 99–104 (2012)
A. Omrani, A.A. Rostami, S. Khostavan, Y. Vazifeshenas, Compos. Part A 43, 381–387 (2012)
A.A. Joraid, A.A. Abu-Sehly, S.N. Alamri, S.Y. Al-Raqa, P.O. Shipman, P.R. Shipley, A.S. Abd-El-Aziz, Thermochim. Acta 529, 22–24 (2012)
J.C. Domínguez, J.C. Grivel, B. Madsen, Thermochim. Acta 529, 29–35 (2012)
R.M. Braga, J.M.F. Barros, D.M.A. Melo, M.A.F. Melo, F.D. Aquino, J.C.D. Freitas, R.C. Santiago, J. Therm, Anal. Calorim. 111(2), 1013–1018 (2013)
G. Rivero, V. Pettarin, A. Vazquez, L.B. Manfredi, Thermochim. Acta 516(1–2), 79–87 (2011)
G.P. Yu, C. Liu, G.H. Li, J.Y. Wang, X.G. Jian, Thermochim. Acta 514(1–2), 51–57 (2011)
Q. Chai, Z.P. Chen, S. Liao, Y. He, Y. Li, W.W. Wu, B. Li, Thermochim. Acta. 533, 74-80 (2012)
Z.P. Chen, Q. Chai, S. Liao, Y. He, Y. Li, X.H. Bo, W.W. Wu, B. Li, Thermochim. Acta 543, 205–210 (2012)
Z.P. Chen, Q. Chai, S. Liao, X. Chen, Y. He, Y. Li, W.W. Wu, B. Li, Ind. Eng. Chem. Res. 51(26), 8985–8991 (2012)
Y. He, S. Liao, Z.P. Chen, Y. Li, Y. Xia, W.W. Wu, B. Li, Ind. Eng. Chem. Res. 52(5), 1870–1876 (2013)
J.E. White, W.J. Catallo, B.L. Legendre, J. Anal, Appl. Pyrolysis. 91(1), 1–33 (2011)
J. Farjas, P. Roura, J. Therm, Anal. Calorim. 105(3), 757–766 (2011)
G.I. Senum, R.T. Yang, J. Therm, Anal. 11(3), 445–447 (1977)
W.J. Tang, Y.W. Liu, H. Zhang, C.X. Wang, Thermochim. Acta 408(1–2), 39–43 (2003)
H.Y. Jiang, J.G. Wang, S.Q. Wu, B.S. Wang, Z.Z. Wang, Carbon 48(2), 352–358 (2010)
S. Vyazovkin, C.A. Wight, In. Rev. Phys. Chem. 17(3), 407–433 (1998)
B. Jankovic, M. Marinovic-Cincovic, V. Jovanovic, S. Samarzija-Jovanovic, G. Markovic, Thermochim. Acta 543, 304–312 (2012)
J. Malek, Thermochim. Acta 200(1–2), 257–269 (1992)
J. Šesták, Academia, Prague, (1984)
C.H. Bamford, C.F.H. Tipper, Elsevier Science Publication, Amsterdam, (1980)
B. Boonchom, J. Therm, Anal. Calorim. 98(3), 863–871 (2009)
B. Boonchom, S. Puttawong, Physica B 405(9), 2350–2355 (2010)
L.T. Vlaev, V.G. Georgieva, S.D. Genieva, J. Therm, Anal. Calorim. 88(3), 805–812 (2007)
X. Gao, D. Dollimore, Thermochim. Acta 215(1–2), 47–63 (1993)
L.T. Vlaev, M.M. Nikolova, G.G. Gospodinov, J. Solid State Chem. 177(8), 2663–2669 (2004)
Z.P. Chen, Q. Chai, S. Liao, Y. He, W.W. Wu, B. Li, J. Therm, Anal. Calorim. 108(3), 1235–1242 (2012)
Y. Li, S. Liao, Z.P. Chen, Y. He, Y. Xia, W.W. Wu, B. Li, Mater. Chem. Phys. 142, 453–458 (2013).
S.C. Turmanova, S.D. Genieva, A.S. Dimitrova, L.T. Vlaev, Express Polym. Lett. 2(2), 133–146 (2008)
A. Ioiţescu, G. Vlase, T. Vlase, N. Doca, J. Therm, Anal. Calorim. 88(1), 121–125 (2007)
T. Vlase, G. Vlase, N. Birta, N. Doca, J. Therm, Anal. Calorim. 88(8), 631–635 (2007)
Acknowledgments
This study was financially supported by the Natural Scientific Foundation of China (Grant No. 21161002), the Dean Project of Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification(Grant No. 2012 K03 and 2012 K07), the Technology the Key laboratory of new processing technology for nonferrous metals and materials, Ministry of Education, Guangxi University (No. GXKFZ-02); the Guangxi Scientific Foundation of China (Grant No. 2012GXNSFAA053019 and No. 0991108); and the Guangxi Science and Technology Agency Research Item of China (Grant No. 0895002–9).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 118 kb)
Appendix
Appendix
Note
Detailed procedure of nonlinear model-fitting method for complex-step reaction:
Assuming the complex-step reaction is combined with two parallel reactions
Where α 1 = x 1 α, α 2 = x 2 α and x 1 + x 2 = 1,which x 1 , x 2 are proportions of reaction 1 and 2 in total reaction. If x 1 < 10 % or x 2 < 10 %, then the process is dominated by a single reaction step, so 0.10 ≤ x 1 ≤ 0.90 or 0.10 ≤ x 2 ≤ 0.90. The rate of the overall transformation process that involves two parallel reactions can be described by Eq. 2.
The procedure performed involved the following steps: (i) Using Kissinger equation (Eq. 4) to estimate A and E α , and N pairs of A and E α are obtained, which are corresponding to N points of α (Δα = 0.02); (ii) The smallest E α and the largest E α combining their corresponding A and T are used as A 1,E α1, T 1, A 2,E α2, T 2; (iii) Using A 1,E α1, T 1, A 2,E α2, T 2 and Eq. 3, calculate k 1(T 1), k 2(T 2); (iv) Using k 1(T 1), k 2(T 2), Eq. 2 and thirty-six models f(α), calculate all possible permutations (dα/dt)calc (Δα = 0.02); (v) Using (dα/dt)calc, (dα/dt)exp (Δα = 0.02), x 1 , x 2 (Δx =0.01) and Eq. 3, calculate all possible permutations RSS; (vi) The smallest RSS and its corresponding A 1,E α1, f 1 (α), x 1 and A 2,E α2, f 2 (α), x 2 are the final results.
Rights and permissions
About this article
Cite this article
Huang, Y., Xia, Y., Liao, S. et al. Kinetics Study with Rigorous Nonlinear Methods for Thermal Decomposition of Polysaccharide Iron Complex. Food Biophysics 9, 277–284 (2014). https://doi.org/10.1007/s11483-014-9351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-014-9351-6