Abstract
Parasite proteins containing repeats are essential invasion ligands, important for their ability to evade the host immune system and to induce immunosuppression. Here, the intrinsic suppressive potential of repetitive structures within parasite proteins was exploited to induce immunomodulation in order to establish self-tolerance in an animal model of autoimmune neurological disease. We tested the tolerogenic potential of fusion proteins containing repeat sequences of parasites linked to self-antigens. The fusion constructs consist of a recombinant protein containing repeat sequences derived from the S-antigen protein (SAg) of Plasmodium falciparum linked to a CD4 T cell epitope of myelin. They were tested for their efficacy to control the development of experimental autoimmune encephalomyelitis (EAE), In addition, we used the DO11.10 transgenic mouse model to study the immune mechanisms involved in tolerance induced by SAg fusion proteins. We found that repeated sequences of P. falciparum SAg protein linked to self-epitopes markedly protected mice from EAE. These fusion constructs were powerful tolerizing agents not only in a preventive setting but also in the treatment of ongoing disease. The tolerogenic effect was shown to be antigen-specific and strongly dependent on the physical linkage of the T cell epitope to the parasite structure and on the action of anti-inflammatory cytokines like IL-10 and TGF-β. Other mechanisms include down-regulation of TNF-α accompanied by increased numbers of FoxP3+ cells. This study describes the use of repetitive structures from parasites linked to defined T cell epitopes as an effective method to induce antigen-specific tolerance with potential applicability for the treatment and prevention of autoimmune diseases.







Similar content being viewed by others
References
Anderton SM, Manickasingham SP, Burkhart C, Luckcuck TA, Holland SJ, Lamont AG, Wraith DC (1998) Fine specificity of the myelin-reactive T cell repertoire: implications for TCR antagonism in autoimmunity. J Immunol 161:3357–3364
Apostolou I, von Boehmer H (2004) In vivo instruction of suppressor commitment in naive T cells. J Exp Med 199:1401–1408
Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F (1999) An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190:995–1004
Bartholomeu DC, de Paiva RM, Mendes TA, DaRocha WD, Teixeira SM (2014) Unveiling the intracellular survival gene kit of trypanosomatid parasites. PLoS Pathog 10:e1004399
Bitar DM, Whitacre CC (1988) Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cell Immunol 112:364–370
Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL (1995) Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376:177–180
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM (2003) Conversion of peripheral CD4 + CD25- naive T cells to CD4 + CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198:1875–1886
Coleman MA, Steptoe RJ (2012) Induction of antigen-specific tolerance through hematopoietic stem cell-mediated gene therapy: the future for therapy of autoimmune disease? Autoimmun Rev 12:195–203
Critchfield JM, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ (1994) T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science 263:1139–1143
Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L (1989) Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol 142:1536–1541
De Trez C, Katsandegwaza B, Caljon G, Magez S (2015) Experimental African trypanosome infection by needle passage or natural tsetse fly challenge thwarts the development of collagen-induced arthritis in DBA/1 prone mice via an impairment of antigen specific B cell autoantibody titers. PLoS One 10:e0130431
Falk K, Rotzschke O, Strominger JL (2000a) Antigen-specific elimination of T cells induced by oligomerized hemagglutinin (HA) 306-318. Eur J Immunol 30:3012–3020
Falk K, Rotzschke O, Santambrogio L, Dorf ME, Brosnan C, Strominger JL (2000b) Induction and suppression of an autoimmune disease by oligomerized T cell epitopes: enhanced in vivo potency of encephalitogenic peptides. J Exp Med 191:717–730
Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF (2004) Cutting edge: TGF-beta induces a regulatory phenotype in CD4 + CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172:5149–5153
Farias AS, Talaisys RL, Blanco YC, Lopes SC, Longhini AL, Pradella F, Santos LM, Costa FT (2011) Regulatory T cell induction during plasmodium chabaudi infection modifies the clinical course of experimental autoimmune encephalomyelitis. PLoS One 6:e17849
Fossati-Jimack L, Ling GS, Baudino L, Szajna M, Manivannan K, Zhao JC, Midgley R, Chai JG, Simpson E, Botto M, Scott D (2015) Intranasal peptide-induced tolerance and linked suppression: consequences of complement deficiency. Immunology 144:149–157
Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A (2005) Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med 11:1118–1124
Gibbon C, Smith T, Egger P, Betts P, Phillips D (1997) Early infection and subsequent insulin dependent diabetes. Arch Dis Child 77:384–385
Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA (2003) CD4 + CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A 100:10878–10883
Gross JA, Callas E, Allison JP (1992) Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol 149:380–388
Gu H, Zou YR, Rajewsky K (1993) Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 73:1155–1164
Gupta S, Pfeil J, Kumar S, Poulsen C, Lauer U, Hamann A, Hoffmann U, Haag R (2015) Tolerogenic modulation of the immune response by oligoglycerol- and polyglycerol-peptide conjugates. Bioconjug Chem 26:669–679
Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P (1983) The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med 157:1149–1169
Higgins PJ, Weiner HL (1988) Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J Immunol 140:440–445
Hong J, Li N, Zhang X, Zheng B, Zhang JZ (2005) Induction of CD4 + CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci U S A 102:6449–6454
Horwitz DA, Zheng SG, Wang J, Gray JD (2008) Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3 + CD4+ Treg. Eur J Immunol 38:912–915
Hughes AL (2004) The evolution of amino acid repeat arrays in plasmodium and other organisms. J Mol Evol 59:528–535
Huntley MA, Golding GB (2004) Neurological proteins are not enriched for repetitive sequences. Genetics 166:1141–1154
Judkowski V, Rodriguez E, Pinilla C, Masteller E, Bluestone JA, Sarvetnick N, Wilson DB (2004) Peptide specific amelioration of T cell mediated pathogenesis in murine type 1 diabetes. Clin Immunol 113:29–37
Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T (2007) Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med 204:57–63
Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, McDevitt HO (1996) Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci U S A 93:3031–3036
Mack J, Falk K, Rotzschke O, Walk T, Strominger JL, Jung G (2001) Synthesis of linear and comb-like peptide constructs containing up to four copies of a T cell epitope and their capacity to stimulate T cells. J Pept Sci 7:338–345
Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK (2014) Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A 112:E156–E165
Maloy KJ, Powrie F (2001) Regulatory T cells in the control of immune pathology. Nat Immunol 2:816–822
Matagne A, Joris B, Frere JM (1991) Anomalous behaviour of a protein during SDS/PAGE corrected by chemical modification of carboxylic groups. Biochem J 280(Pt 2):553–556
Mattsson L, Larsson P, Erlandsson-Harris H, Klareskog L, Harris RA (2000) Parasite-mediated down-regulation of collagen-induced arthritis (CIA) in DA rats. Clin Exp Immunol 122:477–483
Melamed D, Friedman A (1993) Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol 23:935–942
Mendes TA, Lobo FP, Rodrigues TS, Rodrigues-Luiz GF, daRocha WD, Fujiwara RT, Teixeira SM, Bartholomeu DC (2013) Repeat-enriched proteins are related to host cell invasion and immune evasion in parasitic protozoa. Mol Biol Evol 30:951–963
Miller SD, Turley DM, Podojil JR (2007) Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol 7:665–677
Mohammadnia-Afrouzi M, Zavaran Hosseini A, Khalili A, Abediankenari S, Hosseini V, Maleki I (2015) Decrease of CD4 CD25 CD127 FoxP3 regulatory T cells with impaired suppressive function in untreated ulcerative colitis patients. Autoimmunity:1–6
Mohrs K, Harris DP, Lund FE, Mohrs M (2005) Systemic dissemination and persistence of Th2 and type 2 cells in response to infection with a strictly enteric nematode parasite. J Immunol 175:5306–5313
Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC (2013) Regulation of adaptive immunity; the role of interleukin-10. Front Immunol 4:129
O’Farrell AM, Liu Y, Moore KW, Mui AL (1998) IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J 17:1006–1018
Paing MM, Tolia NH (2014) Multimeric assembly of host-pathogen adhesion complexes involved in apicomplexan invasion. PLoS Pathog 10:e1004120
Piaggio E, Mars LT, Cassan C, Cabarrocas J, Hofstatter M, Desbois S, Bergereau E, Rotzschke O, Falk K, Liblau RS (2007) Multimerized T cell epitopes protect from experimental autoimmune diabetes by inducing dominant tolerance. Proc Natl Acad Sci U S A 104:9393–9398
Powrie F, Menon S, Coffman RL (1993) Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol 23:3043–3049
Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL (1996) A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med 183:2669–2674
Puentes F, Dickhaut K, Hofstatter M, Falk K, Rotzschke O (2013) Active suppression induced by repetitive self-epitopes protects against EAE development. PLoS One 8:e64888.
Quintana FJ (2013) Nanoparticles for the induction of antigen-specific Tregs. Immunotherapy 5:437–440
Rashedi I, Panigrahi S, Ezzati P, Ghavami S, Los M (2007) Autoimmunity and apoptosis--therapeutic implications. Curr Med Chem 14:3139–3151
Rotzschke O, Falk K, Strominger JL (1997) Superactivation of an immune response triggered by oligomerized T cell epitopes. Proc Natl Acad Sci U S A 94:14642–14647
Sack BK, Herzog RW, Terhorst C, Markusic DM (2014) Development of Gene transfer for induction of antigen-specific tolerance. Mol Ther Methods Clin Dev 1:14013
Saint RB, Coppel RL, Cowman AF, Brown GV, Shi PT, Barzaga N, Kemp DJ, Anders RF (1987) Changes in repeat number, sequence, and reading frame in S-antigen genes of Plasmodium falciparum. Mol Cell Biol 7:2968–2973
Sakaguchi S (2004) Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562
Shevach EM, Tran DQ, Davidson TS, Andersson J (2008) The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol 38:915–917
Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, Schon MP, Scheffold A, Lowe JB, Hamann A, Syrbe U, Huehn J (2005) Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 106:3097–3104
Stevenson MM, Zavala F (2006) Immunology of malaria infections. Parasite Immunol 28:1–4
Stienekemeier M, Falk K, Rotzschke O, Weishaupt A, Schneider C, Toyka KV, Gold R, Strominger JL (2001) Vaccination, prevention, and treatment of experimental autoimmune neuritis (EAN) by an oligomerized T cell epitope. Proc Natl Acad Sci U S A 98:13872–13877
Tadokoro CE, Vallochi AL, Rios LS, Martins GA, Schlesinger D, Mosca T, Kuchroo VK, Rizzo LV, Abrahamsohn IA (2004) Experimental autoimmune encephalomyelitis can be prevented and cured by infection with Trypanosoma cruzi. J Autoimmun 23:103–115
Targett GA (1992) Virulence and the immune response in malaria. Mem Inst Oswaldo Cruz 87(Suppl 5):137–144
Turley DM, Miller SD (2010) Prospects for antigen-specific tolerance based therapies for the treatment of multiple sclerosis. Results Probl Cell Differ 51:217–235
Vos Q, Lees A, ZQ W, Snapper CM, Mond JJ (2000) B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev 176:154–170
Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH (2009) Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol 183:1577–1586
Weiner HL (2000) Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest 106:935–937
Weiner HL, da Cunha AP, Quintana F, Wu H (2011) Oral tolerance. Immunol Rev 241:241–259
Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM (2005) Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med 202:1199–1212
Yazdanbakhsh M, van den Biggelaar A, Maizels RM (2001) Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol 22:372–377
Zinkernagel RM, Hengartner H (2001) Regulation of the immune response by antigen. Science 293:251–253
Acknowledgments
This work was supported by a Deutsche Forschungsgemeinschaft Grant SFB650. We thank Jorge Enrique Pineda for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Human and Animal Rights
All procedures performed in studies involving animals were in accordance with ethical standards of the Directive 86/609/EEC of the European Community Council and of the institutional, state and federal guidelines. All animal protocols were approved by the ethics committee of the Landesamt für Gesundheit und Soziales (LAGeSo, Berlin, Germany) with registration numbers G0140–06 and G0331–08.
Human Studies
This article does not contain any studies with human participants performed by any of the authors.
Funding
This work was supported by a Deutsche Forschungsgemeinschaft Grant SFB650. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Fabiola Puentes and Katharina Dickhaut contributed equally to the study as shared first author.
Electronic supplementary material
Fig. S1
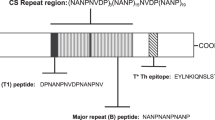
Generation of parasite-derived fusion proteins. (A) Schematic diagram of fusion proteins. Parasite repetitive structures were fused through a linker to CD4+ T cell antigens and expressed in E.coli. Fusion proteins containing repetitive sequences of the S-antigen (SAg) of P. falciparum were designed: 24 repeats of a 8-mer unit derived from the NF7 isolate (A(L/R)KSDEAE) were linked to the N-terminus of the respective CD4+ T cell epitopes: MOG38–51 (SAg-MOG38–51), PLP139–151 (C140S) (SAg-PLP139–151) and OVA323–339 (SAg-OVA323–339). Since the natural units contained either L or R in their repeats, building blocks with alternating amino acids were used in tandem repeats. (B) Analysis of the expression of epitope fusion-constructs containing S-Antigen repeats fused to CD4 T cell epitopes in E. coli. Lane 1: SAg 12-mer (22 KDa); lane 2: SAg 12-mer MOG38–51 S3 (24Kda); lane 3: SAg 12-mer PLP139–151 S3 (24 KDa) and lane M: molecular weight markers. The weights calculated from the DNA sequence are lower compared to those estimated from the SDS gel due to abnormal SDS binding (SAg sequence is 38 % ASP and GLU) (Matagne et al. 1991). (GIF 6 kb)
Fig. S2
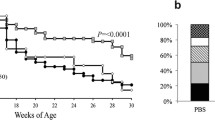
Therapeutic effect of SAg fusion proteins. SJL/J mice (n = 8) were individually treated once the first clinical signs of EAE appeared. 50 μg of SAg-PLP fusion protein was given intravenously to diseased mice. Administration of fusion protein inhibits the evolution of EAE. The curve shows the mean ± SEM daily clinical score. Statistical significance between groups was determined by Mann-Whitney U test. **P < 0.01 and *P < 0.05. (GIF 23 kb)
Fig. S3
Antigen-specific EAE protection after vaccination with SAg-PLP fusion proteins. EAE was induced in SJL/J mice using 50 μg of the encephalitogenic PLP139–151 peptide in CFA. The following day, animals received 200 ng of Pertussis toxin. Mice were vaccinated 7 days prior disease induction with 50 μg of SAg-PLP139–151 or unrelated SAg-OVA323–339 fusion proteins. Mice were monitored daily and the mean clinical score of five mice per group was plotted. One representative experiment of two performed is shown. (GIF 31 kb)
Fig. S4
SAg repeat unit alone does not induce protection in the MOG-EAE model. EAE was induced in C57BL/6 mice by priming with 50 μg of MOG35–55 peptide and injection of 500 ng of Pertussis toxin one day after priming. Mice were vaccinated 7 days before disease induction with 70 μg of SAg-MOG fusion protein or SAg only. Mice were monitored daily and the average ± SEM of the clinical score of five mice per group was calculated. (GIF 30 kb)
Fig. S5
T cell proliferation induced by SAg fusion protein. To compare the proliferative capacity of free peptide and SAg fusion constructs in vitro, [3H]Thymidine proliferation assay was performed. Lymph node cells were harvested from DO11.10 mice and 5x105 cells were incubated for 96 h with titrated amounts of SAg-OVA, free OVA323–339 and MOG38–51 as negative control. [3H]Thymidine was added after 72 h. Counts per minute (cpm) mean values and standard deviation of triplicates are shown. (GIF 21 kb)
Fig. S6
Impact of in vivo neutralization of TGF-β on the protective effect induced by SAg fusion proteins. EAE was induced in SJL/J mice with PLP139–151 peptide. On day 7 after EAE induction, mice received intravenously 50 μg of SAg-PLP or in combination with 0.5 mg of αTGF-β antibody. Control groups were left untreated with or without αTGF-β antibody treatment. Neutralizing antibodies were administered intraperitoneally on days 7, 9 and 11. Animals were scored daily for the development of clinical signs of the disease. Mean clinical score from the groups (n = 5) are presented. (GIF 30 kb)
Fig. S7
Suppressive capacity of SAg constructs is independent of B cells. EAE was induced in JHT+/− and JHT−/− mice after subcutaneous immunization with MOG35–55 peptide emulsified in adjuvant containing Mycobacterium tuberculosis and injection of Pertussis toxin. Mice were treated with SAg-MOG fusion protein and monitored daily. Averages of the clinical scores were calculated. The plot shows that SAg constructs can also induce tolerogenic effect in B cell deficient JHT−/− mice. (GIF 26 kb)
Fig. S8
Antigen-specific suppression of pro-inflammatory TNF-α following treatment with SAg fusion proteins in B cell deficient mice. To measure the production of TNF-α in JHT−/− mice, OTII (OVA323–339 specific) cells labeled with CFSE were transferred into OVA323–339 primed JHT+/− and JHT−/− mice and treated with SAg-OVA. Lymph node cells were isolated from treated and untreated mice and activated for 6 h with OVA323–339 peptide and α-CD28. Cytokine secretion was blocked by the addition of Brefeldin A for the last 4 h of stimulation. Cells were stained for surface CD4 followed by intracellular staining with αTNF-α. For analysis of TNF-α in antigen-specific activated T cells; combined intracellular α-CD154 staining was performed. The frequency of CD154 + TNF-α + double positive in OTII CD4+ T cells was determined and analyzed by FACSDivaTM software (BD Bioscience). (GIF 25 kb)
Fig. S9
Suppressive capacity of SAg-OVA in a DTH model. OVA323–339 specific T cells were isolated from DO11.10 mice and 3x106 CD4+ T cell were adoptively transferred intravenously into Balb/c mice. One day later, mice were tolerized by intravenous administration of 5 μg free OVA323–339 or 60 μg SAg-OVA (equimolar amounts, based on OVA-peptide amount). DTH reaction was induced on day 7 by adoptive transfer of OVA-specific cells that were cultured for 6 days under Th1 polarizing conditions. One day later mice were immunized into the footpad by intradermal injection of 250 ng OVA323–339 in IFA or PBS/ IFA. DTH reaction was measured 8 days post immunization by determining footpad swelling compared to days zero. (GIF 21 kb)
Rights and permissions
About this article
Cite this article
Puentes, F., Dickhaut, K., Hofstätter, M. et al. Immune Modulation and Prevention of Autoimmune Disease by Repeated Sequences from Parasites Linked to Self Antigens. J Neuroimmune Pharmacol 11, 749–762 (2016). https://doi.org/10.1007/s11481-016-9701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9701-x