Abstract
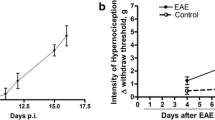
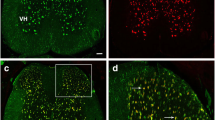
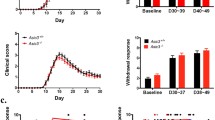
Many multiple sclerosis (MS) patients develop chronic pain, but the underlying pathological mechanism is unknown. Mice with experimental autoimmune encephalomyelitis (EAE) have been widely used to model MS-related neurological complications, including CNS demyelination, neuroinflammation and motor impairments. Similar to MS patients, EAE mice also develop chronic pain. We are interested in elucidating the potential involvement of Wnt signaling in the pathogenesis of chronic pain in EAE mice. In this study, we characterized the expression of Wnt signaling proteins in the spinal cord dorsal horn (SCDH) of EAE mice, by immunoblotting and immunostaining. The EAE model was created by immunization of adult mice (C57BL/6, 10 weeks) with myelin oligodendrocyte glycoprotein (MOG) 35–55. Robust mechanical hyperalgesia and allodynia were developed in both fore- and hindpaws of the EAE mice. Wnt3a, a prototypical Wnt ligand for the canonical pathway, was significantly increased in the SCDH of the EAE mice. Another key protein in the canonical pathway, ß-catenin, was also significantly up-regulated. In addition, Wnt5a, a prototypic Wnt ligand for the non-canonical pathway, and its receptor (co-receptor) Ror2 were also up-regulated in the SCDH of the EAE mice. We further found that Wnt5a antagonist Box5 and β-catenin inhibitor indomethacin attenuated mechanical allodynia in the EAE mice. Our data collectively suggest that Wnt signaling pathways are up-regulated in the SCDH of the EAE mice and that aberrant activation of Wnt signaling contributes to the development of EAE-related chronic pain.






Similar content being viewed by others
References
Aicher SA, Silverman MB, Winkler CW, Bebo BF Jr (2004) Hyperalgesia in an animal model of multiple sclerosis. Pain 110:560–570
Callahan BL, Gil AS, Levesque A, Mogil JS (2008) Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain: Official J Am Pain Soc 9:174–184
Cerpa W, Gambrill A, Inestrosa NC, Barria A (2011) Regulation of NMDA-receptor synaptic transmission by Wnt signaling. The J Neurosci: the Official J Soc Neurosci 31:9466–9471
Chalk JB, McCombe PA, Smith R, Pender MP (1994) Clinical and histological findings in proteolipid protein-induced experimental autoimmune encephalomyelitis (EAE) in the Lewis rat. Distribution of demyelination differs from that in EAE induced by other antigens. J Neurol Sci 123:154–161
Chen J, Park CS, Tang SJ (2006) Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem 281:11910–11916
Chew LJ, Shen W, Ming X, Senatorov VV Jr, Chen HL, Cheng Y, Hong E, Knoblach S, Gallo V (2011) SRY-box containing gene 17 regulates the Wnt/beta-catenin signaling pathway in oligodendrocyte progenitor cells. The J Neurosci: the official J Soc Neurosci 31:13921–13935
Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, Inestrosa NC (2010) Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. The J Neurosci: the official J Soc Neurosci 30:8411–8420
Dal Canto MC, Melvold RW, Kim BS, Miller SD (1995) Two models of multiple sclerosis: experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalomyelitis virus (TMEV) infection. A pathological and immunological comparison. Microsc Res Tech 32:215–229
Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Gene Dev 23:1571–1585
Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH (2011) Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci 14:1009–1016
Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284:15857–15866
Feigenson K, Reid M, See J, Crenshaw EB 3rd, Grinspan JB (2009) Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci 42:255–265
Feigenson K, Reid M, See J, Crenshaw IE, Grinspan JB (2011) Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN neuro 3:e00061
Fradkin LG, Garriga G, Salinas PC, Thomas JB, Yu X, Zou Y (2005) Wnt signaling in neural circuit development. The Journal of neuroscience: the official journal of the Society for Neuroscience 25:10376–10378
Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Grosschedl R (2000) Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 127:469–482
Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI (2009) Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136:1136–1147
Gogolla N, Galimberti I, Deguchi Y, Caroni P (2009) Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron 62:510–525
Jenei V, Sherwood V, Howlin J, Linnskog R, Safholm A, Axelsson L, Andersson T (2009) A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc Natl Acad Sci USA 106:19473–19478
Kiguchi N, Kobayashi Y, Kishioka S (2011) Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol.
Lee HK, Jeong S (2006) β-Catenin stabilizes cyclooxygenase-2 mRNA by interacting with AU-rich elements of 3′-UTR. Nucleic Acids Res 34:5705–5714
Li B, Zhong L, Yang X, Andersson T, Huang M, Tang SJ (2011) WNT5A Signaling contributes to abeta-induced neuroinflammation and neurotoxicity. PloS one 6:e22920
Maguschak KA, Ressler KJ (2012) The dynamic role of beta-catenin in synaptic plasticity. Neuropharmacology 62:78–88
Michalski D, Liebig S, Thomae E, Hinz A, Bergh FT (2011) Pain in patients with multiple sclerosis: a complex assessment including quantitative and qualitative measurements provides for a disease-related biopsychosocial pain model. J Pain Res 4:219–225
Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4:e115
Mikels A, Minami Y, Nusse R (2009) Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem 284:30167–30176
Moore CS, Hebb AL, Blanchard MM, Crocker CE, Liston P, Korneluk RG, Robertson GS (2008) Increased X-linked inhibitor of apoptosis protein (XIAP) expression exacerbates experimental autoimmune encephalomyelitis (EAE). J Neuroimmunol 203:79–93
Murase S, Mosser E, Schuman EM (2002) Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35:91–105
Nuñez F, Bravo S, Cruzat F, Montecino M, De Ferrari GV (2011) Wnt/β-Catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS ONE 6:e18562
Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654
Okuda T, Yu LM, Cingolani LA, Kemler R, Goda Y (2007) beta-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Natl Acad Sci USA 104:13479–13484
Olechowski CJ, Truong JJ, Kerr BJ (2009) Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE). Pain 141:156–164
Olechowski CJ, Parmar A, Miller B, Stephan J, Tenorio G, Tran K, Leighton J, Kerr BJ (2010) A diminished response to formalin stimulation reveals a role for the glutamate transporters in the altered pain sensitivity of mice with experimental autoimmune encephalomyelitis (EAE). Pain 149:565–572
Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J (2002) The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol 31:523–536
Rodrigues DH, Sachs D, Teixeira AL (2009) Mechanical hypernociception in experimental autoimmune encephalomyelitis. Arquivos de neuro-psiquiatria 67:78–81
Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC (2005) Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci 8:34–42
Sahores M, Salinas PC (2011) Activity-mediated synapse formation a role for wnt-fz signaling. Curr Top Dev Biol 97:119–136
Salinas PC, Zou Y (2008) Wnt signaling in neural circuit assembly. Annu Rev Neurosci 31:339–358
Salthouse TN (1964) Luxol fast blue g as a Myelin Stain. Stain Tech 39:123
Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K (2005) Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol 282:397–410
Shubayev VI, Kato K, Myers RR (2010) Cytokines in pain. In: Kruger L, Light AR (eds) Translational pain research: from mouse to man. (Boca Raton, FL
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM (2002) A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 99:467–472
Thibault K, Calvino B, Pezet S (2011) Characterisation of sensory abnormalities observed in an animal model of multiple sclerosis: a behavioural and pharmacological study. Eur J Pain 15:231 e231-216
Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD (2010) NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. The J Neurosci: the Official J Soc Neurosci 30:16383–16390
Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC (2010) Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA 107:21164–21169
Yoshikawa S, McKinnon RD, Kokel M, Thomas JB (2003) Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature 422:583–588
Acknowledgments
This work was supported by the UTMB start-up funds and the Tuberous Sclerosis Alliance to S.-J. T., the National Natural Science Foundation of China (No. 30873457 to L. Z. and the Scientific Technology Project of Guangdong Province of China (No.2008A060202010 and No. 2010B050700019 to L. Z.).
Conflict of Interest Disclaimer
The authors claim that this data set is original and is not under consideration elsewhere. All authors have approved the final version of the manuscript and have no financial or other relationship that might lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, S., Shi, Y. & Tang, SJ. Wnt Signaling in the Pathogenesis of Multiple Sclerosis-Associated Chronic Pain. J Neuroimmune Pharmacol 7, 904–913 (2012). https://doi.org/10.1007/s11481-012-9370-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-012-9370-3