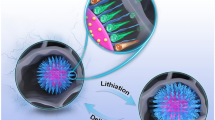
Abstract
Double carbon coated FeP composite (FeP@NC@rGO) was in situ fabricated via the phosphorization process of the as-prepared Prussian blue@graphene oxide (PB@GO) precursor. The FeP nanocrystals were successfully embedded in the nitrogen-doped porous carbon matrix. When used as the anode for lithium ion batteries (LIBs), the FeP@NC@rGO anode shows superior lithium storage properties, delivering a high specific capacity of 830 mA h g−1 after 100 cycles at 100 mA g−1 and excellent rate capability of 359 mA h g−1 at 5 A g−1. The outstanding performance mainly ascribes to the synergistic effect of the double carbon coating and porous structure design. The introduction of porous carbon and graphene coating on FeP nanoparticles greatly enhance the electronic conductivity of the active material and well accommodates the large volume variation of FeP during the cycling process.
Similar content being viewed by others
References
Ohzuku T, Makimura Y. Chem Lett, 2001, 30: 642–643
Xu K. Chem Rev, 2004, 104: 4303–4418
Derrien G, Hassoun J, Panero S, Scrosati B. Adv Mater, 2007, 19: 2336–2340
Liu C, Li F, Ma LP, Cheng HM. Adv Mater, 2010, 22: E28–E62
Guo YG, Chen J. Sci China Chem, 2017, 60: 1–2
Shin HC, Liu M. Adv Funct Mater, 2005, 15: 582–586
Wang Y, Xu M, Peng Z, Zheng G. J Mater Chem A, 2013, 1: 13222–13226
Park CM, Kim JH, Kim H, Sohn HJ. Chem Soc Rev, 2010, 39: 3115–3141
Liu Q, Tian J, Cui W, Jiang P, Cheng N, Asiri AM, Sun X. Angew Chem, 2014, 126: 6828–6832
Yuan D, Huang G, Yin D, Wang X, Wang C, Wang L. ACS Appl Mater Interfaces, 2017, 9: 18178–18186
Yuan C, Wu HB, Xie Y, Lou XWD. Angew Chem Int Ed, 2014, 53: 1488–1504
Li Q, Li Z, Zhang Z, Li C, Ma J, Wang C, Ge X, Dong S, Yin L. Adv Energy Mater, 2016, 6: 1600376
Wu C, Kopold P, van Aken PA, Maier J, Yu Y. Adv Mater, 2017, 29: 1604015
Fan M, Chen Y, Xie Y, Yang T, Shen X, Xu N, Yu H, Yan C. Adv Funct Mater, 2016, 26: 5002
Cui YH, Xue MZ, Fu ZW, Wang XL, Liu XJ. Cheminform, 2013, 44: 283–290
Veluri PS, Mitra S. Cheminform, 2016, 47: 87675–87679
Jiang H, Chen B, Pan J, Li C, Liu C, Liu L, Yang T, Li W, Li H, Wang Y, Chen L, Chen M. J Alloys Compd, 2017, 728: 328–336
Wang X, Na Z, Yin D, Wang C, Huang G, Wang L. Energy Storage Mater, 2018, 12: 103–109
Xin S, Guo YG, Wan LJ. Acc Chem Res, 2012, 45: 1759–1769
Yang ZD, Chang ZW, Xu JJ, Yang XY, Zhang XB. Sci China Chem, 2017, 60: 1540–1545
Jiang J, Wang C, Liang J, Zuo J, Yang Q. Dalton Trans, 2015, 44: 10297–10303
Zhang XD, Shi JL, Liang JY, Yin YX, Guo YG, Wan LJ. Sci China Chem, 2017, 60: 1554–1560
Wang X, Chen K, Wang G, Liu X, Wang H. ACS Nano, 2017, 11: 11602–11616
Yang W, Hao J, Zhang Z, Zhang B. J Colloid Interface Sci, 2015, 460: 55–60
Zhang JW, Zhang HT, Du ZY, Wang X, Yu SH, Jiang HL. Chem Commun, 2013, 50: 1092–1094
Kaye SS, Dailly A, Yaghi OM, Long JR. J Am Chem Soc, 2007, 129: 14176–14177
Zhang W, Ma D, Du J. Talanta, 2014, 120: 362–367
Kong B, Selomulya C, Zheng G, Zhao D. Chem Soc Rev, 2015, 44: 7997–8018
Karyakin AA, Puganova EA, Budashov IA, Kurochkin IN, Karyakina EE, Levchenko VA, Matveyenko VN, Varfolomeyev SD. Anal Chem, 2004, 76: 474–478
DeLongchamp D, Hammond P. Adv Funct Mater, 2004, 14: 224–232
Zhao W, Xu JJ, Shi CG, Chen HY. Langmuir, 2005, 21: 9630–9634
Uemura T, Ohba M, Kitagawa S. Inorg Chem, 2004, 43: 7339–7345
Graham HD. J Agric Food Chem, 1992, 40: 801–805
Zhu X, Liu M, Liu Y, Chen R, Nie Z, Li J, Yao S. J Mater Chem A, 2016, 4: 8974–8977
Hummers WS Jr., Offeman RE. J Am Chem Soc, 1958, 80: 1339
Zhang L, Wu HB, Madhavi S, Hng HH, Lou XWD. J Am Chem Soc, 2012, 134: 17388–17391
Liu X, Zhong X, Yang Z, Pan F, Gu L, Yu Y. Electrochim Acta, 2015, 152: 178–186
Grosvenor AP, Wik SD, Cavell RG, Mar A. Inorg Chem, 2005, 44: 8988–8998
Stankovich S, Piner RD, Chen X, Wu N, Nguyen SBT, Ruoff RS. J Mater Chem, 2006, 16: 155–158
Wang S, Chen M, Xie Y, Fan Y, Wang D, Jiang JJ, Li Y, Grützmacher H, Su CY. Small, 2016, 12: 2365–2375
Liu X, Cheng J, Li W, Zhong X, Yang Z, Gu L, Yu Y. Nanoscale, 2014, 6: 7817–7822
Liu J, Xu Y, Ma X, Feng J, Qian Y, Xiong S. Nano Energy, 2014, 7: 52–62
Boyanov S, Bernardi J, Gillot F, Dupont L, Womes M, Tarascon JM, Monconduit L, Doublet ML. Cheminform, 2006, 37: 3531–3538
Li Z, Li B, Yin L, Qi Y. ACS Appl Mater Interfaces, 2014, 6: 8098–8107
Xu YT, Guo Y, Li C, Zhou XY, Tucker MC, Fu XZ, Sun R, Wong CP. Nano Energy, 2015, 11: 38–47
Liu X, Wu Y, Yang Z, Pan F, Zhong X, Wang J, Gu L, Yu Y. J Power Sources, 2015, 293: 799–805
Guo P, Song H, Chen X. Electrochimica Acta, 2009, 11: 1320–1324
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFB0100305), the National Natural Science Foundation of China (51622210), and the Fundamental Research Funds for the Central Universities (WK3430000004).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11426_2018_9278_MOESM1_ESM.docx
FeP Nanoparticles Derived from Metal-Organic Frameworks/GO and Phosphorization as High-Performance Anode Material for Lithium Ion Batteries
Rights and permissions
About this article
Cite this article
Gao, M., Liu, X., Yang, H. et al. FeP nanoparticles derived from metal-organic frameworks/GO as high-performance anode material for lithium ion batteries. Sci. China Chem. 61, 1151–1158 (2018). https://doi.org/10.1007/s11426-018-9278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-018-9278-5