Abstract
N-Benzyl-substituted 2C class phenethylamines (NBOMes) are psychoactive designer drugs, with strong hallucinogenic and stimulant effects, even at low doses. The designer drug, 2-(4-bromo-2, 5-dimethoxyphenyl)-N-(2-methoxybenzyl) ethanamine (25B-NBOMe) is considered to be one of the most potent agonists of the serotonin-2A (5-HT2A) receptor. Recently, we reported the first lethal case of 25B-NBOMe intoxication with severe rhabdomyolysis, concluded by clinical, pathological and toxicological analyses. There are currently no good animal models that closely recapitulate serotonin receptor-dependent rhabdomyolysis. In the present study, we created animal models of rhabdomyolysis using zebrafish larvae to study the pathomechanism of rhabdomyolysis, and demonstrated that 25B-NBOMe can simulate lethal rhabdomyolysis in this animal. Treatment of the larvae with 25B-NBOMe decreased their survival rate, locomotion, altered birefringence of the skeletal muscle and immunostainings for dystroglycan (a myoseptal protein) and myosin heavy chain (a myofibril protein), which were consistent with rhabdomyolysis. This 25B-NBOMe-induced rhabdomyolysis was inhibited by the 5-HT2A receptor antagonists ritanserin and aripirazole, but not by the 5-HT1A + 5-HT1B receptor antagonist propranolol and the 5-HT3 receptor antagonist granisetron, indicating 5-HT2A-dependent rhabdomyolysis. The 25B-NBOMe-treated zebrafish is, therefore, a highly useful model of rhabdomyolysis for studying the pathomechanism of rhabdomyolysis as well as for therapeutic drug screening.
Similar content being viewed by others
Introduction
Serotonin syndrome is caused by the excessive activation of serotonin (5-hydroxytryptamine: 5-HT) receptors in the nervous system, and is characterized by autonomic hyperactivity, mental-status changes, and neuromuscular abnormalities including rhabdomyolysis [1, 2]. Serotonin syndrome is known to be induced not only by diverse serotonergic drugs [i.e., monoamine oxidase (MAO) inhibitors, selective serotonin reuptake inhibitors (SSRIs) and the 5-HT precursor (l-tryptophan)] and their combinations, but also by illegal drugs such as methylenedioxymethamphetamine (MDMA) [3]. In rodent models, a combination of serotonergic drugs (precursors) can induce serotonin receptor 2A (5-HT2A)-dependent hyperthermia [4]. However, to our knowledge, there has been no reproducible animal model of serotonin syndrome with overt rhabdomyolysis established to date.
N-2-Methoxy-benzyl substituted 2C class hallucinogens (NBOMes) had emerged as psychoactive designer drugs that potently activated 5-HT2A receptors [5]. NBOMe poisoning can cause various symptoms that are similar to serotonin syndrome, including tachycardia, hypertension, agitation, hallucinations, seizures, hyperpyrexia, and myoclonus [6]. Because 45% of the lethal cases of serotonin syndrome presented high serum levels of creatine kinase, rhabdomyolysis may be a predominant cause of death in NBOMe intoxication [7]. Recently, we reported a case of serotonin syndrome with lethal rhabdomyolysis after the ingestion of 2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl) ethanamine (25B-NBOMe), one of the most potent 5-HT2A agonists with α1-adrenoceptor agonist activity [8]. Prominent rhabdomyolysis was also reported in two other cases with severe 25B-NBOMe intoxication [9]. Because the patho-physiological mechanism underlying rhabdomyolysis associated with serotonin syndrome is largely unknown, we hypothesized that 25B-NBOMe will contribute to the study of the mechanism of 5-HT2A-dependent rhabdomyolysis.
Zebrafish have been widely used in neuroscience researches due to their high genetic homology to humans, genetic tractability, low cost, and, hence, usefulness in high-throughput analyses [10]. Serotonergic drugs and their combinations can induce serotonin syndrome-like behavior such as surface dwelling and hypo-locomotion in zebrafish, in association with high serotonin levels in the brain [10]. In zebrafish larvae, the structures of organs, such as the heart and skeletal muscle, can be observed in situ due to their transparency and small size. Furthermore, they can be analysed in a short period and in large scale owing to their easy maintenance and housing, fast growth, and high fecundity. Because of the in vivo visibility of their skeletal muscle and easy genetic manipulation, zebrafish has been used to study muscular dystrophy [11–13] as well as for therapeutic drug screening [14–16].
Here, we present a novel, simple and reproducible model of 5-HT2A-dependent rhabdomyolysis induced by 25B-NBOMe in zebrafish.
Materials and methods
Chemicals
The 25B-NBOMe hydrochloride was purchased from Cayman chemical (Ann Arbor, MI, USA); ritanserin, aripiprazole, granisetron hydrochloride, and propranolol hydrochloride from Sigma-Aldrich (St. Louis, MO, USA). Other common chemicals used were of the highest purity commercially available.
Fish and fish culture
The Experimental Animal Committee of Tokyo Medical University approved all experiments performed in this study (approval number: S28029). Adult zebrafish (the AB line) strains of both sexes were obtained from the Aquatic Resources Program (Boston Children’s Hospital, Boston, MA, USA) and acclimatized to the laboratory environment for at least 14 days in a 100-L aquarium filled with continuously unchlorinated water at 28.5 °C, with constant filtration and density of up to five animals per liter according to the standard procedures [17] and standard criteria [18]. The animals were kept on a day/night cycle of 12/12 h and fed twice a day with flaked fish food. Fertilized eggs were collected and cultured for drug treatments.
Drug treatment of zebrafish
Pairs of adult AB zebrafish were mated, and their embryos were cultured to larvae 4 days post fertilization (dpf). Five-4-dpf larvae were put into one well (a total of three wells for each condition) and were treated with either 0, 0.005, 0.5, or 5 μg/mL of 25B-NBOMe [stock solution: 10 mg/mL in dimethyl sulfoxide (DMSO)] for 2 days to determine the effective concentration of 25B-NBOMe. The survival rates of 25B-NBOMe-treated and vehicle (DMSO)-treated fish were analyzed. All experiments were repeated three times.
To analyze the 5-HT receptor subtype responsible for effects, zebrafish larvae were treated with 25B-NBOMe (0.5 μg/mL) and 5-HT receptor subtype inhibitors including ritanserin (1.0 μM), aripiprazole (0.5 μM), granisetron hydrochloride (100 μM), or propranolol hydrochloride (10 μM) for 2 days. The concentration of each inhibitor was set at the maximum concentration required to enable the survival of larvae for 2 days. Ritanserin is a selective 5-HT2A + 5-HT2C receptor antagonist; aripirazole is a 5-HT2A receptor antagonist, and partial 5-HT1A + dopamine D2 receptor agonist; granisetron hydrochloride is a 5-HT3 receptor antagonist; propranolol hydrochloride is a 5-HT1A + 5-HT1B antagonist and non-selective β-adrenoreceptor (β-AR) blocker. For the specificity of 5-HT subtype receptors, see review [19]. All experiments were repeated four times.
Detection of muscle structural changes by the birefringence assay
To monitor structural changes in skeletal muscle, birefringence was examined using a dissection microscope (MZ10F; Leica Microsystems GmbH, Wetzlar, Germany). Zebrafish larvae were anesthetized with 0.32% tricaine solution, placed on a polarizing filter and covered with a second polarizing filter. The filters were placed on a stereomicroscope and the top-polarizing filter was twisted until only the light refracting through the striated muscle became visible. As the degree of birefringence is affected by the horizontal orientation of the fish, the fish were oscillated back and forth to account for differences in positioning. Muscle damage was detected as reduced birefringence.
Immunohistochemistry
For immunohistochemical staining, whole larvae were fixed in 4% paraformaldehyde overnight at 4 °C and stored in 100% methanol at −20 °C. Following rehydration with a 50% methanol solution in phosphate buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) and blocking with 2% casein in PBS-T to reduce non-specific immunoreactions, larvae were incubated with either anti-beta dystroglycan (1:100, Novocastra; Leica Biosystems, Wetzlar, Germany) or anti-myosin heavy chain (MHC) (F59, 1:25; Santa Cruz Biotechnology, Dallas, TX, USA) antibodies at 4 °C overnight. After washing several times, the larvae were incubated with a secondary antibody (1:500, anti-mouse AlexaFluor 488; Thermo Fisher Scientific, Waltham, MA, USA) for 30 min at room temperature. The stained larvae were then observed using a confocal microscope (LCM710; Carl Zeiss Microscopy GmbH, Jena, Germany).
Behavioral analysis of zebrafish larvae
Each zebrafish larva of 4 dpf was put into each well of a 96-well plate containing the drugs and their swimming behavior was recorded for 5 min using Danio Vision (Noldus, Wageningen, The Netherlands). The swimming distance of each fish was measured using Danio Vision following the manufacturer’s instructions. Eight larvae were examined for each condition.
Reverse transcription polymerase chain reaction
To confirm the expression of the 5-HT2A receptor, zebrafish total RNA was extracted from the brain and skeletal muscle of adult zebrafish (3-month old) using the RNeasy micro kit (Qiagen, Venlo, The Netherlands) and was converted to cDNA (Superscript III; Thermo Fisher Scientific). To detect PCR products of 5-HT2A receptor cDNA, PCR was performed using ExTaq DNA Polymerase (Takara Bio, Kusastu, Japan) at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s for 35 cycles) with the following primer sets [20]: zebrafish 5-HT2A receptor, forward 5′-GCCACCAATTACTTCCTCATGTCAC-3′, reverse 5′-GGTTCACAAACCACTGCCAAAAC-3′; and β-actin, forward: 5′-ATCAGCATGGCTTCTGCTCT-3′, reverse: 5′-CACCCTGGCTTACATTTTCAA-3′. The resulting DNAs were subjected to gel electrophoresis for visualization of DNA bands corresponding to 5-HT2A receptor and β-actin [20].
Results
Reduction of zebrafish larva survival by 25B-NBOMe
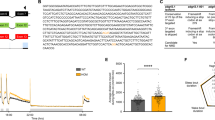
From 4 dpf, zebrafish larvae were treated with 25B-NBOMe at the concentrations of 0, 0.005, 0.5, or 5 μg/mL for 2 days. Treatment with 25B-NBOMe was found to decrease the survival rate of zebrafish in a dose-dependent manner (Fig. 1a). Treatment with 0.5 μg/mL of 25B-NBOMe resulted in a reduction in survival rate to about 60% of the control fish (untreated: 93.3 ± 9.4, 0.005% DMSO: 95.6 ± 8.3%, 25B-NBOMe at 0.5 μg/mL: 58.3 ± 15.2%). Therefore, in subsequent experiments, we used 25B-NBOMe at 0.5 μg/mL (Fig. 1a, p = 0.00000458).
a Survival rates of zebrafish larvae as a function of 25B-NBOMe concentrations, treated or untreated for 2 days. The asterisk shows the significant difference between vehicle and 0.5 μg/mL 25B-NBOMe groups (p = 0.00000458). b Microscopic photographs of zebrafish larvae untreated and treated with 0.5 μg/mL 25B-NBOMe. c Ratio of zebrafish larvae with reduced muscle birefringence (BR) after treatment with 0.5 μg/mL 25B-NBOMe and no treatment
Muscle degeneration in 25B-NBOMe-treated larvae
Two days after the treatment of zebrafish larvae with 0.5 μg/mL of 25B-NBOMe, the ratio of live larvae with reduced birefringence was 65–80% (25B-NBOMe at 0.5 μg/mL: 66.0 ± 20%, Fig. 1c). Immunofluorescence with an anti-beta dystroglycan antibody demonstrated irregularity and defects of the myosepta, whereas immunofluorescence with an anti-MHC antibody demonstrated myofibril injury (Fig. 2). These structural changes in the skeletal muscle after 25B-NBOMe-treatment were consistent with rhabdomyolysis.
Microscopic immunofluorescent photographs after immunostaining for β-dystroglycan and myosin heavy chain in skeletal muscle of zebrafish larvae with and without treatment by 25B-NBOMe at 0.5 μg/mL for 2 days. The arrowhead and arrow in the right upper panel show irregularity and disruption of the myosepta, respectively. The arrows shown in the right lower panel show myofibril injuries inflicted by 25B-NBOMe. The bar shows the length of 50 μm
5-HT2A receptor antagonists prevented 25B-NBOMe-induced hypo-locomotion, death and muscle injury
Zebrafish larvae were co-treated with 25B-NBOMe (0.5 μg/mL) and a 5-HT subtype inhibitor for 2 days. The 5-HT2A receptor antagonists, aripiprazole and ritanserin, significantly improved the survival rate of the 25B-NBOMe-treated larvae (*p = 0.032 and **p = 0.013, respectively). However, the 5-HT1A + 5-HT1B antagonist (β-AR blocker) propranolol, and the 5-HT3 receptor antagonist granisetron did not affect the survival of the 25B-NBOMe-treated larvae (Fig. 3a). The increase in the ratio of larvae with reduced birefringence by 25B-NBOMe-treatment were significantly reduced by aripiprazole or ritanserin (*p = 0.023 and **p = 0.013, respectively) (Fig. 3b). These findings indicated that 5-HT2A receptor was involved in rhabdomyolysis induced by 25B-NBOMe. Behavioral analysis demonstrated that ritanserin, but not aripiprazole or granisetron, significantly reduced 25B-NBOMe-induced hypo-locomotion (reduced swimming distance) (*p = 0.023) (Fig. 3c). On the other hand, propranolol enhanced the 25B-NBOMe-induced hypo-locomotion (**p = 0.020). Expression of the 5-HT2A receptor in zebrafish skeletal muscle was confirmed by reverse transcription polymerase chain reaction (Fig. 3d).
Effects pf some 5-HT receptor inhibitors in the presence of 0.5 μg/mL 25B-NBOMe on a survival rate, b ratio of zebrafish with reduced muscle BR and c locomotion of zebrafish larvae (*p = 0.032, **p = 0.013 versus no inhibitor for a; *p = 0.023, **p = 0.013 versus no inhibitor for b; *p = 0.023, **p = 0.020 versus no inhibitor for c). The DNA profile for 5-HT2A receptor and β-actin obtained from the brain and skeletal muscle of adult zebrafish using gel electrophoresis is also shown in d
Discussion
In zebrafish larvae, 25B-NBOMe, one of the most potent 5-HT2A agonists known to date, induced lethal rhabdomyolysis (Fig. 1a). The rhabdomyolysis was confirmed not only by the reduction in muscle birefringence (Fig. 1c), but also by the reduced immunostaining for a sarcolemmal (myoseptal) protein (β-dystroglycan) and myofibril protein in skeletal muscle (Fig. 2). The 25B-NBOMe-induced rhabomyolysis was prevented by treatment with either aripiprazole or ritanserin (5-HT2A antagonists), but not by propranolol (5-HT1A + 5-HT1B antagonist) or granisetron (5-HT3 antagonist). These findings confirmed the induction of 5-HT2A-dependent rhabdomyolysis by 25B-NBOMe-treatment. However, according to a review on 5-HT receptors [19], the 5-HT2A receptor is implicated in the contraction of smooth muscle, but the presence of 5-HT2A receptors in skeletal muscle was not mentioned.
In the skeletal muscle of young and adult rats, 5-HT2A receptors were shown to localize to the sarcolemma and T-tubules, respectively [21]. In zebrafish muscle, however, the localization of 5-HT2A could not be analyzed, because there were no anti-5-HT2A antibodies available with reactivity to the zebrafish epitope. Instead, we could confirm the presence of a 5-HT2A-receptor gene in the zebrafish (Fig. 3d).
In rodent skeletal muscle, it was shown that 5-HT2A activation contributed to muscle differentiation and glycolysis. Via 5-HT2A, 5-HT induced the transcriptional activation of myogenin and glucose transporter 3, thereby promoting muscle differentiation and glycolysis, respectively [22]. Additionally, 5-HT was shown to activate the key glycolytic enzyme 6-phosphofructo-1-kinase [23]. The activation of glycolysis can enhance muscle contraction via an increase in intracellular adenosine triphosphate (ATP) and Ca2+ levels. In cardiomyogenic cells cultured in a high glucose medium, we demonstrated that hypoxia induces excessive glycolysis accompanied by metabolic acidosis (excessive intracellular H+), an increase in intracellular Na+ via the Na+/H+-exchanger, an increase in intracellular Ca2+ via the Na+/Ca2+-exchanger, and finally cell death via the Ca2+-dependent protease calpain [24]. It remains to be clarified as to whether 25B-NBOMe causes an over-activation of glycolysis and increases intracellular ATP and Ca2+ levels, resulting in rhabdomyolysis.
Muscle hypertonicity and hyperthermia are predominant manifestations of serotonin syndrome, reflecting rhabdomyolysis in general [2], which is induced by 25B-NBOMe [7, 8]. Additionally, a few studies have suggested that 5-HT2A stimulation enhances muscle contraction under particular conditions. In spinal cord injury, persistent inward Ca2+ currents induce muscle spasms via the activation of 5-HT2 and α1-adrenergic receptors [25], which can be activated also by 25B-NBOMe [8]. In excitable cells, 5-HT and the serotonergic drug MDMA modulates Ca2+-driven signals through the coupling of L-type Ca2+-channels and serotonin transporters [26]. Given its potent 5-HT2A agonistic effects [5], 25B-NBOMe may induce intracellular Ca2+ overload and skeletal muscle over-contraction, in association with rhabdomyolysis. The latter possibility remains to be addressed.
Rhabdomyolysis occurs not only in serotonin syndrome, but also in malignant hyperthermia (MH). MH is characterized by severe hyperthermia and rhabdomyolysis via excessive sarcoplasmic reticulum Ca2+ release [27]. Similarly to anesthetics, the 5-HT2A agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride induced rapid and intense contraction in muscle isolated from MH patients, compared with that from healthy volunteers [28], and the hyper-contraction was prevented by ritanserin [27]. In the well-established rat model of serotonin syndrome, co-administration of 5-hydroxy-l-tryptophan (the 5-HT precursor) and clorgyline (a MAO inhibitor) induced lethal hyperthermia, which was prevented by 5-HT2A antagonists (ritanserin and pipamperone), but not by the 5-HT1A + 5-HT1B antagonist propranolol [29], as we found in this zebrafish model of rhabdomyolysis induced by 25B-NBOMe (Fig. 3a–c). On the other hand, fluoxetine (an SSRI) suppresses impaired muscle birefringence in a zebrafish model of Duchenne muscular dystrophy, suggesting the implication of serotonin reuptake in dystrophic muscle [16]. Collectively, we hypothesized that excessive serotonin uptake and 5-HT2A-activation may generally induce muscle degeneration including rhabdomyolysis.
Stewart et al. [10] proposed that zebrafish provide a promising model to investigate serotonin syndrome due to genetic homology to humans, genetic tractability, and low cost of maintenance. Our zebrafish model of rhabdomyolysis with 25B-NBOMe has many advantages over the frequently utilized rat model. In the rat model, rhabdomyolysis can only be analyzed by body temperature and creatine kinase release into the blood. In the zebrafish model, however, we can directly observe and analyze the temporal progression of muscle degeneration by the birefringence assay due to their transparency. Additionally, whole-body immunofluorescence for myosepta and myofibril proteins visualized the disruption of the two structures by a simple procedure. Moreover, due to the small sizes and large numbers of available fish, high-throughput analyses can be performed under diverse conditions in a short period of time, at low costs, with minimal effort, and with small amounts of drugs and fewer animals.
In zebrafish, various behavioral parameters of psychiatric disorders, including those due to drug abuse, can be temporally recorded for high-throughput, unbiased and semi-quantitative analyses using video-tracking technologies [10]. Stewart et al. [10] proposed that surface dwelling and hypo-locomotion in zebrafish after the administration of serotonergic drugs represented serotonin syndrome. Consistently, we confirmed 25B-NBOMe-induced hypo-locomotion in our zebrafish larva model. This hypo-locomotion was prevented by the 5-HT2A antagonist ritanserin, but not by the 5-HT2A antagonist aripiprazole (Fig. 3c). Given that the two 5-HT2A antagonists prevented the 25B-NBOMe-induced reduction in muscle birefringence to a similar extent (Fig. 3b), this indicates that muscle weakness does not induce hypo-locomotion. Differences in the effects of the two 5-HT2A antagonists on 25B-NBOMe-induced hypo-locomotion may be due to the fact that ritaserin, but not aripirazole, can inhibit 5-HT2C and 5-HT5 receptors which affect locomotion [19]. On the other hand, Wappler et al. [30] proposed that serotonin syndrome is induced by overstimulation of the central nervous system 5-HT1A receptor by high synaptic 5-HT levels, in association with its interaction with 5-HT2A and dopaminergic receptors. However, the non-specific 5-HT1A antagonist propranolol did not prevent, but rather aggravated the 25B-NBOMe-induced hypo-locomotion (Fig. 3c). Therefore, although it has been believed that rhabdomyolysis is one of the diverse symptoms of serotonin syndrome, our study suggests that rhabdomyolysis and behavioral manifestations of serotonin syndrome are mediated by different 5-HT receptor subtypes and pathways.
Conclusions
In recent years, zebrafish have been emerging as a useful animal model in various fields of the neurosciences, including physiology, pharmacology, toxicology and even psychiatry, because the small fish have various advantages such as their high homology to humans, genetic tractability, low cost and high-throughput analyses. In this study, we presented a simple and reproducible model of rhabdomyolysis induced by 25B-NBOMe, a potent hallucinogenic designer drug, using zebrafish larvae, which indicated that the 5-HT2A receptor was involved in the formation of rhabdomyolysis. The zebrafish have a high potential to be utilized for assessing the pharmacological, neurobehavioral and toxic efforts of novel psychotropic substances, and for studies on the mechanisms of their effect manifestation in forensic toxicology.
Change history
24 August 2017
An erratum to this article has been published.
References
Werneke U, Jamshidi F, Taylor DM, Ott M (2016) Conundrums in neurology: diagnosing serotonin syndrome—a meta-analysis of cases. BMC Neurol 16:97. doi:10.1186/s12883-016-0616-1
Boyer EW, Shannon M (2005) The serotonin syndrome. N Engl J Med 352:1112–1120
Warren JD, Blumbergs PC, Thompson PD (2002) Rhabdomyolysis: a review. Muscle Nerve 25:332–347
Shioda K, Nisijima K, Yoshino T, Kato S (2010) Mirtazapine abolishes hyperthermia in an animal model of serotonin syndrome. Neurosci Lett 482:216–219
Kyriakou C, Marinelli E, Frati P, Santurro A, Afxentiou M, Zaami S, Busardo FP (2015) NBOMe: new potent hallucinogens–pharmacology, analytical methods, toxicities, fatalities: a review. Eur Rev Med Pharmacol Sci 19:3270–3281
Hill SL, Doris T, Gurung S, Katebe S, Lomas A, Dunn M, Blain P, Thomas SH (2013) Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clin Toxicol 51:487–492
Suzuki J, Dekker MA, Valenti ES, Cruz FAA, Correa AM, Poklis JL, Poklis A (2015) Toxicities associated with NBOMe ingestion—a novel class of potent hallucinogens: a review of the literature. Psychosomatics 56:129–139
Yoshida K, Saka K, Shintani-Ishida K, Maeda M, Nakajima N, Hara S, Ueno M, Sasaki M, Iwase H, Sakamoto T (2015) A case of fatal intoxication due to the new designer drug 25B-NBOMe. Forensic Toxicol 33:396–401
Tang MH, Ching CK, Tsui MS, Chu FK, Mak TW (2014) Two cases of severe intoxication associated with analytically confirmed use of the novel psychoactive substances 25B-NBOMe and 25C-NBOMe. Clin Toxicol 52:561–565
Stewart AM, Cachat J, Gaikwad S, Robinson KS, Gebhardt M, Kalueff AV (2013) Perspectives on experimental models of serotonin syndrome in zebrafish. Neurochem Int 62:893–902
Bassett DI, Currie PD (2003) The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum Mol Genet 12:265–270
Guyon JR, Goswami J, Jun SJ, Thorne M, Howell M, Pusack T, Kawahara G, Steffen LS, Galdzicki M, Kunkel LM (2009) Genetic isolation and characterization of a splicing mutant of zebrafish dystrophin. Hum Mol Genet 18:202–211
Kawahara G, Guyon JR, Nakamura Y, Kunkel LM (2010) Zebrafish models for human FKRP muscular dystrophies. Hum Mol Genet 19:623–633
Siebel AM, Vianna MR, Bonan CD (2014) Pharmacological and toxicological effects of lithium in zebrafish. ACS Chem Neurosci 5:468–476
Kawahara G, Karpf JA, Myers JA, Alexander MS, Guyon JR, Kunkel LM (2011) Drug screening in a zebrafish model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 108:5331–5336
Waugh TA, Horstick E, Hur J, Jackson SW, Davidson AE, Li X, Dowling JJ (2014) Fluoxetine prevents dystrophic changes in a zebrafish model of Duchenne muscular dystrophy. Hum Mol Genet 23:4551–4562
Nusslein-Volhard C, Dahm R (2002) Zebrafish: a practical approach. Oxford University Press, Oxford
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310
Pytliak M, Vargová V, Mechírová V, Felšöci M (2011) Serotonin receptors—from molecular biology to clinical applications. Physiol Res 60:15–25
Sourbron J, Schneider H, Kecskés A, Liu Y, Buening EM, Lagae L, Smolders I, de Witte P (2016) Serotonergic modulation as effective treatment for Dravet syndrome in a zebrafish mutant model. ACS Chem Neurosci 7:588–598
Hajduch E, Dombrowski L, Darakhshan F, Rencurel F, Marette A, Hundal HS (1999) Biochemical localisation of the 5-HT2A (serotonin) receptor in rat skeletal muscle. Biochem Biophys Res Commun 257:369–372
Guillet-Deniau I, Burnol AF, Girard J (1997) Identification and localization of a skeletal muscle secrotonin 5-HT2A receptor coupled to the Jak/STAT pathway. J Biol Chem 272:14825–14829
Coelho WS, Costa KC, Sola-Penna M (2007) Serotonin stimulates mouse skeletal muscle 6-phosphofructo-1-kinase through tyrosine-phosphorylation of the enzyme altering its intracellular localization. Mol Genet Metab 92:364–370
Aki T, Yoshida K, Fujimiya T (2002) Phosphoinositide 3-kinase accelerates calpain-dependent proteolysis of fodrin during hypoxic cell death. J Biochem 132:921–926
D’Amico JM, Murray KC, Li Y, Chan KM, Finlay MG, Bennett DJ, Gorassini MA (2013) Constitutively active 5-HT2/α1 receptors facilitate muscle spasms after human spinal cord injury. J Neurophysiol 109:1473–1484
Ruchala I, Cabra V, Solis E Jr, Glennon RA, De Felice LJ, Eltit JM (2014) Electrical coupling between the human serotonin transporter and voltage-gated Ca2+ channels. Cell Calcium 56:25–33
Wappler F, Scholz J, Fiege M, Richter A, Steinfath M, Weisshorn R, Schulte am Esch J (1999) 5-HT2 receptor antagonist-mediated inhibition of halothane-induced contractures in skeletal muscle specimens from malignant hyperthermia susceptible patients. Naunyn Schmiedebergs Arch Pharmacol 360:376–381
Gerbershagen MU, Wappler F, Fiege M, Kolodzie K, Weisshorn R, Szafarczyk W, Kudlik C, Schulte Am Esch J (2003) Effects of a 5HT2 receptor agonist on anaesthetized pigs susceptible to malignant hyperthermia. Br J Anaesth 91:281–284
Nisijima K, Yoshino T, Yui K, Katoh S (2001) Potent serotonin (5-HT)2A receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 890:23–31
Wappler F, Fiege M, Schulte am Esch J (2001) Pathophysiological role of the serotonin system in malignant hyperthermia. Br J Anaesth 87:794–798
Acknowledgements
This work was supported partly by a Grant-in-Aid for Challenging Exploratory Research, Scientific Research (B) and (C) from the Japan Society for the Promotion of Science, by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, by Research on Rare and Intractable Diseases, Health and Labor Science Research Grants, and by an Intramural Research Grant for Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry (26-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Ethical approval
The use of zebrafish for this study was approved by the Experimental Animal Committee of Tokyo Medical University (approval number: S28029). This article does not contain any studies with human participants performed by any of the authors.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s11419-017-0383-8.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kawahara, G., Maeda, H., Kikura-Hanajiri, R. et al. The psychoactive drug 25B-NBOMe recapitulates rhabdomyolysis in zebrafish larvae. Forensic Toxicol 35, 369–375 (2017). https://doi.org/10.1007/s11419-017-0366-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-017-0366-9