Abstract
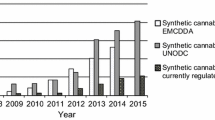
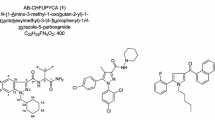
Recently, a large number of synthetic cannabinoids have been identified in herbal mixtures. Moreover, an even higher number of cannabimimetic compounds are currently distributed as research chemicals on a gram to kilogram scale via several online trading platforms. As this situation leads to a large number of new cannabimimetics and the occurrence of isobaric substances, the analysis of such compounds using mass spectroscopy (MS) involves the risk of incorrect assignments of mass spectra. In certain cases, this leads to considerable analytical challenges. In the majority of cases, these challenges can only be mastered by combining multiple analytical techniques. We purchased a so-called research chemical advertised as the cannabimimetic compound [(N-methylpiperidin-2-yl)methyl]-3-(1-naphthoyl)indole (AM-1220) via an Internet platform. Analysis of the microcrystalline substance using gas chromatography (GC)–MS indicated the presence of pure AM-1220. However, after further purity testing utilizing thin-layer chromatography we were surprised to see an additional spot indicating a mixture of two substances with highly similar physicochemical properties. After isolation, high-resolution mass spectroscopy (HR-MS) revealed an elemental composition of C26H26N2O for both substances, proving the presence of two isobaric substances. Moreover, GC–MS and LC-HR-MS/MS experiments indicated two naphthoylindoles featuring different heterocyclic substituents at the indole nitrogen. Nuclear magnetic resonance spectroscopy verified the presence of the highly potent cannabimimetic AM-1220 and its azepane isomer. Interestingly, only a few weeks after purchasing the powder we also detected both substances in a similar proportion in several herbal mixtures for the first time.





Similar content being viewed by others
References
Synthetic Cannabinoids in Herbal Products. United Nations Office on Drugs and Crime (2011), UN Document ID number: SCITEC/24
Kneisel S, Westphal F, Rösner P, Brecht V, Ewald A, Klein B, Pütz M, Thiemt S, Auwärter V (2011) Cannabimimetics: mass spectra and IR-ATR spectra of new compounds from the years 2009 and 2010. TIAFT Bull 41(1):38–48
Kneisel S, Westphal F, Moosmann B, Brecht V, Bisel P, Vidal C, Jacobsen-Bauer A, Bork W-R, Auwärter V (2011) Cannabimimetics II: mass spectra and ATR-IR spectra of new compounds between the end of 2010 and late 2011. TIAFT Bull 41(3):29–38
Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N (2009) ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom 44:832–837
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (2009) Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol 27:61–66
Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T (2009) Spice: a never ending story? Forensic Sci Int 191:58–63
Westphal F, Junge T, Sönnichsen F, Rösner P, Schäper J (2010) Ein neuer Wirkstoff in SPICE-artigen Kräutermischungen: Charakterisierung von JWH-250, seinen Methyl- und Trimethylsilylderivaten. Toxichem Krimtech 77(1):8–22
Westphal F, Sönnichsen FD, Thiemt S (2011) Identification of 1-butyl-3-(1-(4-methyl)naphthoyl)indole in a herbal mixture. Forensic Sci Int. doi:10.1016/j.forsciint.2011.03.031
Ernst L, Schiebel H-M, Theuring C, Lindigkeit R, Beuerle T (2011) Identification and characterization of JWH-122 used as new ingredient in “Spice-like” herbal incenses. Forensic Sci Int 208:e31–e35
Bononi M, Belgi P, Tateo F (2011) Analytical data for identification of the cannabimimetic phenylacetylindole JWH-203. J Anal Toxicol 35:360–363
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2011) Identification and quantitation of two cannabimimetic phenylacetylindoles JWH-251 and JWH-250, and four cannabimimetic naphthoylindoles JWH-081, JWH-015, JWH-200, and JWH-073 as designer drugs in illegal products. Forensic Toxicol 29:25–37
Uchiyama N, Kikura-Hanajiri R, Goda Y (2011) Identification of a novel cannabimimetic phenylacetylindole, cannabipiperidiethanone, as a designer drug in a herbal product and its affinity for cannabinoid CB1 and CB2 receptors. Chem Pharm Bull 59:1203–1205
Nakajima J, Takahashi M, Nonaka R, Seto T, Suzuki J, Yoshida M, Kanai C, Hamano T (2011) Identification and quantitation of a benzoylindole (2-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone and a naphthoylindole 1-(5-fluoropentyl-1H-indol-3-yl)-(naphthalene-1-yl) ethanone (AM-2201) found in illegal products obtained via the Internet and their cannabimimetic effects evaluated by in vitro [35S]GTPγS binding assays. Forensic Toxicol. doi:10.1007/s11419-011-0114-5
Nakajima J, Takahashi M, Seto T, Kanai C, Suzuki J, Yoshida M, Hamano T (2011) Identification and quantitation of two benzoylindoles AM-694 and (4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone, and three cannabimimetic naphthoylindoles JWH-210, JWH-122, and JWH-019 as adulterants in illegal products obtained via the Internet. Forensic Toxicol. doi:10.1007/s11419-011-0108-3
Jankovics P, Váradi A, Tölgyesi L, Lohner S, Németh-Palotás J, Balla J (2011) Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary. Forensic Sci Int. doi:10.1016/j.forsciint.2011.07.011
Kneisel S, Westphal F, Bisel P, Brecht V, Broecker S, Auwärter V (2012) Identification and structural characterization of the synthetic cannabinoid 3-(1-adamantoyl)-1-pentylindole as an additive in ‘herbal incense’. J Mass Spectrom. doi:10.1002/jms.2059
Moosmann B, Kneisel S, Girreser U, Brecht V, Westphal F, Auwärter V (2012) Separation and structural characterisation of the synthetic cannabinoids JWH-412 and 1-[(5-fluoropentyl)-1H-indol-3yl[-(4-methylnaphthalen-1-yl)methanone using GC-MS, NMR analysis and a flash chromatography system. Forensic Sci Int. doi:10.1016/j.forsciint.2011.12.010
(2011) EMCDDA reporting form on the new drug AM-2232. Forensic Toxicology Department, University Medical Center Freiburg. Accessible via EDND database of the EMCDDA
Hudson S, Ramsey J, King L, Timbers S, Maynard S, Dargan PI, Wood DM (2010) Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol 34:252–260
D’Ambra TE, Eissenstat MA, Abt J, Ackerman JH, Bacon ER, Bell MR, Carabateas PM, Josef KA, Kumar V, Weaver JD, Arnold R, Casiano FM, Chippari SM, Haycock DA, Kuster JE, Luttinger DA, Stevenson JI, Ward SJ, Hill WA, Khanolkar A, Makriyannis A (1996) C-Attached aminoalkylindoles: potent cannabinoid mimetics. Bioorg Med Chem Lett 6:17–22
Deng H, Gifford AN, Zvonok AM, Cui G, Li X, Fan P, Deschamps JR, Flippen-Anderson JL, Gatley SJ, Makriyannis A (2005) Potent cannabinergic indole analogues as radioiodinatable brain imaging agents for the CB1 cannabinoid receptor. J Med Chem 48:6386–6392
Makriyannis A, Deng H (2008) Cannabimimetic indole derivatives. United States Patent US 2008/0090871 A1
Willis PG, Pavlova OA, Chefer SI, Vaupel DB, Mukhin AG, Horti AG (2005) Synthesis and structure–activity relationship of a novel series of aminoalkylindoles with potential for imaging the neuronal cannabinoid receptor by positron emission tomography. J Med Chem 48:5813–5822
Acknowledgments
We thank the EU Commission (JUST/2009/DPIP/AG/0948), the German Ministry of Health, and the City of Frankfurt (Main) for funding the project "Spice and synthetic cannabinoids". Parts of this article have been published in the members’ journal of the German Society of Toxicological and Forensic Chemistry (GTFCh) as well as in the Bulletin of TIAFT [3]. We thank Prof. Torsten Arndt (Ingelheim, Germany) as well as Dr. Dimitri Gerostamoulos and Dr. Jochen Beyer (Melbourne, Australia) for kindly lifting the copyright.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kneisel, S., Bisel, P., Brecht, V. et al. Identification of the cannabimimetic AM-1220 and its azepane isomer (N-methylazepan-3-yl)-3-(1-naphthoyl)indole in a research chemical and several herbal mixtures. Forensic Toxicol 30, 126–134 (2012). https://doi.org/10.1007/s11419-012-0137-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-012-0137-6