Abstract
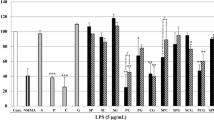
During the development of natural herbal medicines in Japan, Glehniae Radix cum Rhizoma (Hamabofu in Japanese) has been used as a substitute for Saposhnikoviae Radix (Bofu). Bofu and Hamabofu are blended differently in several Kampo formulae. For example, Bofu is included in Jumihaidokuto by a manufacturer, whereas Hamabofu is included instead of Bofu in the same formula by other manufacturers. Although both Bofu and Hamabofu are used for their expected anti-inflammatory effects, differences in their medicinal properties are not well characterized. In addition, there have been very few reports comparing the pharmacological activities of the constituents in Bofu and Hamabofu. In the present study, we investigated the anti-inflammatory effects of the extracts of Bofu and Hamabofu by monitoring levels of the inflammatory mediator nitric oxide (NO) produced in rat hepatocytes. Moreover, the chemical constituents responsible for the activity were investigated. Our results showed that ethyl acetate fractions of Bofu and Hamabofu extracts contain different compounds, although both fractions suppressed NO production in rat hepatocytes. The linear dihydropyranochromones from the Bofu extract (i.e., 3′-O-angeloylhamaudol, ledebouriellol and hamaudol) suppressed NO production, whereas the coumarins from the Hamabofu extract (i.e., umbelliferone and scopoletin) also suppressed NO production. These results suggest that linear dihydropyranochromones and coumarins are responsible for the anti-inflammatory effects of Bofu and Hamabofu. It is plausible that Bofu and Hamabofu are blended differently in several Kampo formulae due to many constituents with as yet unidentified pharmacological activity.




Similar content being viewed by others
References
Li L, Li B, Zhang H, Zhao A, Han B, Liu C, Tsao R (2015) Ultrafiltration LC-ESI-MS screening of MMP-2 inhibitors from selected Chinese medicinal herbs Smilax glabra Roxb., Smilax china L. and Saposhnikovia divaricata (Turcz.) Schischk as potential functional food ingredients. J Funct Food 15:389–395
Yen KY (1992) The Illustrated Chinese materia medica: crude and prepared. SMC, Taipei, Taiwan, pp 1–340
Wang CC, Chen LG, Yang LL (1999) Inducible nitric oxide synthase inhibitor of the Chinese herb I. Saposhnikovia divaricata (Turcz.) Schischk. Cancer Lett 145:151–157
Su X, Li X, Tao H, Zhou J, Wu T, Chou G, Cheng Z (2013) Simultaneous isolation of seven compounds from Glehnia littoralis roots by off-line overpressured layer chromatography guided by a TLC antioxidant autographic assay. J Sep Sci 36:3644–3650
Ng TB, Liu F, Wang HX (2004) The antioxidant effects of aqueous and organic extracts of Panax quinquefolium, Panax notoginseng, Codonopsis pilosula, Pseudostellaria heterophylla, and Glehnia littoralis. J Ethnopharmacol 93:285–288
Kong CS, Um YR, Lee JI, Yea SS, Seo YW (2010) Constituents isolated from Glehnia littoralis suppress proliferations of human cancer cells and MMP expression in HT1080 cells. Food Chem 120:385–394
Yuan Z, Tezuka Y, Fan WZ, Kadota S, Li X (2002) Constituents of the underground parts of Glehnia littoralis. Chem Pharm Bull 50:73–77
Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi M, Yamazaki M, Hosokawa M, Hiraoka N, Anetai M, Masuda T, Takasugi M (1998) Analgesic components of Glehnia root (Glehnia littoralis). J Nat Med 52:491–501
Kitade H, Sakitani K, Inoue K, Masu Y, Kawada N, Hiramatsu Y, Kamiyama Y, Okumura T, Ito S (1996) Interleukin 1β markedly stimulates nitric oxide formation in the absence of other cytokines or lipopolysaccharide in primary cultured rat hepatocytes but not in Kupffer cells. Hepatology 23:797–802
Colasanti R, Suzuki H (2000) The dual personality of NO. Trends Pharmacol Sci 13:249–252
Matsui K, Nishizawa M, Ozaki T, Kimura T, Hashimoto I, Yamada M, Kaibori M, Kamiyama Y, Ito S, Okumura T (2008) Natural antisense transcript stabilizes inducible nitric oxide synthase messenger RNA in rat hepatocytes. Hepatology 47:686–997
Takimoto Y, Qian HY, Yoshigai E, Okumura T, Ikeya Y, Nishizawa M (2013) Gomisin N in the herbal drug gomishi (Schisandra chinensis) suppresses inducible nitric oxide synthase gene via C/EBPβ and NF-κB in rat hepatocytes. Nitric Oxide 28:47–56
Nakajima A, Yamamoto Y, Yoshinaka N, Namba M, Matsuo H, Okuyama T, Yoshigai E, Okumura T, Nishizawa M, Ikeya Y (2015) A new flavanone and other flavonoids from green perilla leaf extract inhibit nitric oxide production in interleukin 1β-treated hepatocytes. Biosci Biotechnol Biochem 79:138–146
Inaba H, Yoshigai E, Okuyama T, Murakoshi M, Sugiyama K, Nishino H, Nishizawa M (2015) Antipyretic analgesic drugs have different mechanisms for regulation of the expression of inducible nitric oxide synthase in hepatocytes and macrophages. Nitric Oxide 44:61–70
Kanemaki T, Kitade H, Hiramatsu Y, Kamiyama Y, Okumura T (1993) Stimulation of glycogen degradation by prostaglandin E2 in primary cultured rat hepatocytes. Prostaglandins 45:459–474
Green LC, Wanger DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biol Chem 126:131–138
Sasaki H, Taguchi H, Endo T, Yoshioka I (1982) The constituents of Ledebouriella seseloides WOLFF. I. Structures of three new chromones. Chem Pharm Bull 30:3555–3562
Park Y, Kim H, Lee S, Choi H, Jin C, Lee Y (2007) A new chromone, 11-Hydroxy-sec-O-glucosylhamaudol from Ostericum koreanum. Chem Pharm Bull 55:1065–1066
Ruo Y, Zhong Y, Yang L (2008) Cytotoxic phenylpropanoids from carrot. J Agric Food Chem 56:3024–3027
Harker S, Razdan TK, Waight ES (1984) Steroids, chromenes and coumarins from Angelica officinalis. Phytochemistry 23:419–426
Kang J, Sun JH, Zhou L, Ye M, Han J, Wang BR, Guo DA (2008) Characterization of compounds from the roots of Saposhnikovia divaricata by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 22:1899–1911
Yang W, Ye M, Liu M, Kong D, Shi R, Shi X, Zhang K, Wang Q, Lantong Z (2010) A practical strategy for the characterization of coumarins in Radix Glehniae by liquid chromatography coupled with triple quadrupole-linear ion trap mass spectrometry. J Chromatogr A 1217:4587–4600
Oda K, Yamaguchi Y, Yoshimura T, Wada K, Nishizono N (2007) Synthetic models related to furanocoumarin-CYP3A4 interactions. comparison of furanocoumarin, coumarin, and benzofuran dimers as potent inhibitors of CYP3A4 activity. Chem Pharm Bull 55:1419–1421
Yamada H, Hanawa T, Kim S (2012) Kampo medical and pharmaceutical sciences for pharmacy student. Nankodo, Tokyo, pp 344–365
Acknowledgments
We would like to thank Dr. Yukinobu Ikeya for his technical advice, and Ms. Kana Nakao for her technical support. This work was supported by a Grant-in-Aid for Promotion of International Research, Ritsumeikan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamino, T., Shimokura, T., Morita, Y. et al. Comparative analysis of the constituents in Saposhnikoviae Radix and Glehniae Radix cum Rhizoma by monitoring inhibitory activity of nitric oxide production. J Nat Med 70, 253–259 (2016). https://doi.org/10.1007/s11418-016-0969-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-0969-1