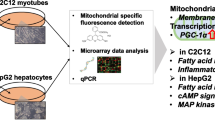
Abstract
Enhancement of muscular energy production is thought to improve locomotive functions and prevent metabolic syndromes including diabetes and lipidemia. Black ginger (Kaempferia parviflora) has been cultivated for traditional medicine in Thailand. Recent studies have shown that black ginger extract (KPE) activated brown adipocytes and lipolysis in white adipose tissue, which may cure obesity-related dysfunction of lipid metabolism. However, the effect of KPE on glucose and lipid utilization in muscle cells has not been examined yet. Hence, we evaluated the effect of KPE and its constituents on energy metabolism in pre-differentiated (p) and differentiated (d) C2C12 myoblasts. KPE (0.1–10 μg/ml) was added to pC2C12 cells in the differentiation process for a week or used to treat dC2C12 cells for 24 h. After culturing, parameters of glucose and lipid metabolism and mitochondrial biogenesis were assessed. In terms of the results, KPE enhanced the uptake of 2-deoxyglucose and lactic acid as well as the mRNA expression of glucose transporter (GLUT) 4 and monocarboxylate transporter (MCT) 1 in both types of cells. The expression of peroxisome proliferator-activated receptor γ coactivator (PGC)-1α was enhanced in pC2C12 cells. In addition, KPE enhanced the production of ATP and mitochondrial biogenesis. Polymethoxy flavonoids in KPE including 5-hydroxy-7-methoxyflavone, 5-hydroxy-3,7,4′-trimethoxyflavone and 5,7-dimethoxyflavone enhanced the expression of GLUT4 and PGC-1α. Moreover, KPE and 5,7-dimethoxyflavone enhanced the phosphorylation of 5′AMP-activated protein kinase (AMPK). In conclusion, KPE and its polymethoxy flavonoids were found to enhance energy metabolism in myocytes. KPE may improve the dysfunction of muscle metabolism that leads to metabolic syndrome and locomotive dysfunction.







Similar content being viewed by others
References
Hepple RT (2014) Mitochondrial involvement and impact in aging skeletal muscle. Front Aging Neurosci 6:211. doi:10.3389/fnagi.2014.00211
Nair KS (2005) Aging muscle. Am J Clin Nutr 81:953–963
Rooyackers OE, Adey DB, Ades PA, Nair KS (1996) Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93:15364–15369
Barazzoni R, Short KR, Nair KS (2000) Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 275:3343–3347
Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C (2006) Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 5:179–195
Dillon LM, Rebelo AP, Moraes CT (2012) The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life 64:231–241
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142
Montessuit C, Rosenblatt-Velin N, Papageorgiou I, Campos L, Pellieux C, Palma T, Lerch R (2004) Regulation of glucose transporter expression in cardiac myocytes: p38 MAPK is a strong inducer of GLUT4. Cardiovasc Res 64:94–104
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017
Juel C, Halestrap AP (1999) Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. J Physiol 517:633–642
Halestrap AP, Price NT (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343:281–299
dos Santos JM, Benite-Ribeiro SA, Queiroz G, Duarte JA (2012) The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle. Cell Biochem Funct 30:191–197
Akase T, Shimada T, Terabayashi S, Ikeya Y, Sanada H, Aburada M (2011) Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J Nat Med 65:73–80
Shimada T, Horikawa T, Ikeya Y, Matsuo H, Kinoshita K, Taguchi T, Ichinose K, Takahashi K, Aburada M (2011) Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia 82:1272–1278
Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon SJ (2005) Anti-gastric ulcer effect of Kaempferia parviflora. Ethno pharmacol 102:120–122
Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, Lailerd N, Suttajit M, Jaikang C, Chaiyasut C (2009) Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med Chem 5:139–147
Chaturapanich G, Chaiyakul S, Verawatnapakul V, Yimlamai T, Pholpramool C (2012) Enhancement of aphrodisiac activity in male rats by ethanol extract of Kaempferia parviflora and exercise training. Andrologia 44:323–328
Chaipech S, Morikawa T, Ninomiya K, Yoshikawa M, Pongpiriyadacha Y, Hayakawa T, Muraoka O (2012) Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem Pharm Bull 60:62–69
Sutthanut K, Sripanidkulchai B, Yenjai C, Jay M (2007) Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J Chromatography A 1143:227–233
Murata K, Hayashi H, Matsumura S, Matsuda H (2013) Suppression of benign prostate hyperplasia by Kaempferia parviflora rhizome. Pharmacognosy Res 5:309–314
Yenjai C, Wanich S, Pitchuanchom S, Sripanidkulchai B (2009) Structural modification of 5,7-dimethoxyflavone from Kaempferia parviflora and biological activities. Arch Pharm Res 32:1179–1184
Patanasethanont D, Nagai J, Matsuura C, Fukui K, Sutthanut K, Sripanidkulchai BO, Yumoto R, Takano M (2007) Modulation of function of multidrug resistance associated-proteins by Kaempferia parviflora extracts and their components. Eur J Pharmacol 566:67–74
Sae-Wong C, Matsuda H, Tewtrakul S, Tansakul P, Nakamura S, Nomura Y, Yoshikawa M (2011) Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J Ethnopharm 136:488–495
Lee YS, Cha BY, Saito K, Yamakawa H, Choi SS, Yamaguchi K, Yonezawa T, Teruya T, Nagai K, Woo JT (2010) Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem Pharmacol 79:1674–1683
Matsushita M, Yoneshiro T, Aita S, Kamiya T, Kusaba N, Yamaguchi K, Takagaki K, Kameya T, Sugie H, Saito M (2015) Kaempferia parviflora extract increases whole-body energy expenditure in humans. Roles of brown adipose tissue. J Nutr Sci Vitaminol 61:79–83 (Tokyo)
Promthep K, Eungpinichpong W, Sripanidkulchai B, Chatchawan U (2015) Effect of Kaempferia parviflora extract on physical fitness of soccer players. A randomized double-blind placebo-controlled trial. Med Sci Monit Basic Res 21:100–108
Yoshino S, Kim M, Awa R, Kuwahara H, Kano Y, Kawada T (2014) Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci Nutr 2:634–637
Okabe Y, Shimada T, Horikawa T, Kinoshita K, Koyama K, Ichinose K, Aburada M, Takahashi K (2014) Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine 21:800–806
Montessuit C, Rosenblatt-Velin N, Papageorgiou I, Campos L, Pellieux C, Palma T, Lerch R (2004) Regulation of glucose transporter expression in cardiac myocytes: p38 MAPK is a strong inducer of GLUT4. Cardiovasc Res 64:94–104
Schiaffino S, Mammucari C (2011) Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1(1):4. doi:10.1186/2044-5040-1-4
Handschin C, Spiegelman BM (2011) PGC-1 coactivators and the regulation of skeletal muscle fiber-type determination. Cell Metabol 13:351
Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, Criswell DS (2010) Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol 588(Pt 18):3551–3566
Irrcher I, Ljubicic V, Kirwan AF, Hood DA (2008) AMP-activated protein kinase-regulated activation of the PGC-1α promoter in skeletal muscle cells. PLoS ONE 3(10):e3614. doi:10.1371/journal.pone.0003614
Kim MS, Hur HJ, Kwon DY, Hwang JT (2012) Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol Cell Endocrinol 358:127–134
Tsutsumi R, Yoshida T, Nii Y, Okahisa N, Iwata S, Tsukayama M, Hashimoto R, Taniguchi Y, Sakaue H, Hosaka T, Shuto E, Sakai T (2014) Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr Metab (Lond) 4(11):32. doi:10.1186/1743-7075-11-32
Matsuda H, Kogami Y, Nakamura S, Sugiyama T, Ueno T, Yoshikawa M (2011) Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. Bioorg Med Chem 19:2835–2841
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to be declared by the authors.
Rights and permissions
About this article
Cite this article
Toda, K., Takeda, S., Hitoe, S. et al. Enhancement of energy production by black ginger extract containing polymethoxy flavonoids in myocytes through improving glucose, lactic acid and lipid metabolism. J Nat Med 70, 163–172 (2016). https://doi.org/10.1007/s11418-015-0948-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-015-0948-y