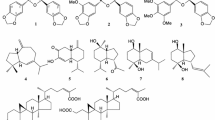
Abstract
A methanol extract of the flower buds of Chimonanthus praecox (L.) Link (Calycanthaceae) demonstrated inhibitory effects on melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells. From the extract, five dimeric pyrrolidinoindoline alkaloids and four sesquiterpenes were isolated, together with 16 known compounds. Among them, (−)-chimonanthine (1, IC50 = 0.93 μM), (−)-folicanthine (2, 1.4 μM), and (−)-calycanthidine (3, 1.8 μM) showed potent inhibitory effects without notable cytotoxicity at the effective concentrations. The most potent alkaloid (1) inhibited both tyrosinase and tyrosine-related protein-1 mRNA expressions, to which the melanogenesis inhibitory activity would be ascribable.


Similar content being viewed by others
References
Zhang J-W, Gao J-M, Xu T, Zhang X-C, Ma Y-T, Jarussophon S, Konishi Y (2009) Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem Biodiv 6:838–845
Wang W-X, Cao L, Xiong J, Xia G, Hu J-F (2011) Constituents from Chimonanthus praecox (wintersweet). Phytochem Lett 4:271–274
Lv J-S, Zhang L-L, Chu X-Z, Zhou J-F (2012) Chemical composition, antioxidant and antimicrobial activity of the extracts of the flowers of the Chinese plant Chimonanthus praecox. Nat Prod Res 26:1363–1367
Takayama H, Matsuda Y, Masubuchi K, Ishida A, Kitajima M, Aimi N (2004) Isolation, structure elucidation, and total synthesis of two new Chimonanthus alkaloids, chimonamidine and chimonanthidine. Tetrahedron 60:893–900
Kitajima M, Mori I, Arai K, Kogure N, Takayama H (2006) Two new tryptamine-derived alkaloids from Chimonanthus praecox f. concolor. Tetrahedron Lett 47:3199–3202
Prota G (1988) Progress in the chemistry of melanins and related metabolites. Med Res Rev 8:525–556
Kim YJ, Uyama H (2005) Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 62:1707–1723
Hearing VJ, Korner AM, Pawelek JM (1982) New regulators of melanogenesis are associated with purified tyrosinase isozymes. J Invest Dermatol 79:16–18
Hearing VJ, Jiménez M (1987) Mammalian tyrosinase-the critical regulatory control point in melanocyte pigmentation. Int J Biochem 19:1141–1147
Kuzumaki T, Matsuda A, Wakamatsu K, Ito S, Ishikawa K (1993) Eumelanin biosynthesis is regulated by coordinate expression of tyrosinase and tyrosinase-related protein-1 genes. Exp Cell Res 207:33–40
Friedmann PS, Gilchrest BA (1987) Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol 133:88–94
Hunt G, Todd C, Cresswell JE, Thody AJ (1994) Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci 107:205–211
Steinberg ML, Whittaker JR (1976) Stimulation of melanotic expression in a melanoma cell line by theophylline. J Cell Physiol 87:265–275
Wong G, Pawelek J (1975) Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in Cloudman S91 melanoma cells. Nature 255:644–646
Matsuda H, Morikawa T, Ninomiya K, Yoshikawa M (2001) Hepatoprotective constituents from Zedoariae rhizome: absolute stereostructures of three new carabrane-type sesquiterpenes, curcumenolactiones A, B, and C. Bioorg Med Chem 9:909–916
Raharivelomanana P, Bianchini J-P, Cambon A, Azzaro M, Faure R (1995) Two-dimensional NMR of sesquiterpenes 8—complete assignment of 1H and 13C NMR spectra of seven sesquiterpene alcohols from Neocallitropsis pancheri. Magn Reson Chem 33:233–235
Matsuda H, Kageura T, Oda M, Morikawa T, Sakamoto Y, Yoshikawa M (2001) Effects of constituents from the bark of Magnolia obovata on nitric oxide production in lipopolysaccharide-activated macrophages. Chem Pharm Bull 49:716–720
Kitagawa I, Cui Z, Son BW, Kobayashi M, Kyogoku Y (1987) Marine natural products. XVII. Nephtheoxydiol, a new cytotoxic hydroperoxy-germacrane sesquiterpene, and related sesquiterpenoids from an Okinawan soft coral of Nephthea sp. (Nephtheidae). Chem Pharm Bull 35:124–135
Kim JS, Kim JC, Shim SH, Lee EJ, Jin WY, Bae K, Son KH, Kim HP, Kang SS, Chang HW (2006) Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch Pharm Res 29:617–623
Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi M, Yamazaki M (2001) Analgesic components of saposhnikovia root (Saposhnikovia divaricata). Chem Pharm Bull 49:154–160
Voirin S, Baumes R, Bayonove C (1990) Synthesis and n.m.r. spectral properties of grape monoterpenyl glycosides. Carbohydr Res 207:39–56
Otsuka H, Takeda Y, Yamasaki K (1990) Xyloglucosides of benzyl and phenethyl alcohols and Z-hex-3-en-1-ol from leaves of Alangium platanifolium var. trilobum. Phytochemistry 29:3681–3683
Hamerski L, Bomm MD, Silva DHS, Young MCM, Furlan M, Eberlin MN, Castro-Gamboa I, Cavalheiro AJ, da Silva Bolzani V (2005) Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 66:1927–1932
Konishi T, Wada S, Kiyosawa S (1993) Constituents of the leaves of Daphne pseudo-mezereum. Yakugaku Zasshi 113:670–675
Bisset NG, Choudhury AK, Houghton PJ (1989) Phenolic glycosides from the fruit of Strychnos nux-vomica. Phytochemistry 28:1553–1554
Chaurasia N, Wichtl M (1987) Flavonolglykoside aus Urtica dioica. Planta Med 53:432–434
Senatore F, D’Agostino M, Dini I (2000) Flavonoid glycosides of Barbarea vulgaris L. (Brassicaceae). J Agric Food Chem 48:2659–2662
De Rosa S, De Giulio A, Tommonaro G (1996) Aliphatic and aromatic glycosides from the cell cultures of Lycopersicon esculentum. Phytochemistry 42:1031–1034
Verotta L, Orsini F, Sbacchi M, Scheildler MA, Amador TA, Elisabetsky E (2002) Synthesis and antinociceptive activity of chimonanthines and pyrrolidinoindoline-type alkaloids. Bioorg Med Chem 10:2133–2142
Fang C-L, Horne S, Taylor N, Rodrigo R (1994) Dimerization of a 3-substituted oxindole at C-3 and its application to the synthesis of (±)-folicanthine. J Am Chem Soc 116:9480–9486
Hu F, Lesney PF (1964) The isolation and cytology of two pigment cell strains from B-16 mouse melanomas. Cancer Res 24:1634–1643
Nakashima S, Matsuda H, Oda Y, Nakamura S, Xu F, Yoshikawa M (2010) Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg Med Chem 18:2337–2345
Bao K, Dai Y, Zhu Z-B, Tu F-J, Zhang W-G, Yao X-S (2010) Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorg Med Chem 18:6708–6714
Parvez S, Kang M, Chung HS, Bae H (2007) Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res 21:805–816
Parvez S, Kang M, Chung H-S, Cho C, Hong M-C, Shin M-K, Bae H (2006) Survey and mechanism of skin depigmentating and lightening agents. Phytother Res 20:921–934
Chang T-S (2009) An updated review of tyrosinase inhibitors. Int J Mol Sci 10:2440–2475
Bertolotto C, Buscá R, Abbe P, Bille K, Aberdam E, Ortonne J-P, Ballotti R (1998) Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol Cell Biol 18:694–702
Conflict of interest
The authors have declared that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morikawa, T., Nakanishi, Y., Ninomiya, K. et al. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox . J Nat Med 68, 539–549 (2014). https://doi.org/10.1007/s11418-014-0832-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-014-0832-1