Abstract
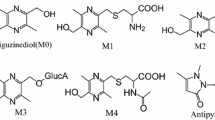
This experiment's with aim was to study the pharmacokinetics of asperosaponin VI and its three metabolites (cauloside A, HN saponin F and hederagenin) via a sensitive high performance liquid chromatography connected with electrospray ionization triple quadrupole mass spectrum (HPLC–ESI–MS/MS). Chromatographic separation was achieved on a reverse phase C18 column with a gradient mobile phase of CH3CN-water with 0.1 % HCOOH at a flow rate of 0.3 mL/min. Sample analysis was simultaneously performed with a multiple reaction monitoring mode using target determination ions at m/z 927.5 → 603.4 for asperosaponin VI, m/z 811.1 → 603.4 for cauloside A, m/z 649.4 → 603.4 for HN saponin F, m/z 71.4 → 393.3 for hederagenin and m/z 307.0 → 161.1 for warfarin as the internal standard. The calibration curve was linear at the range of 0.25–500 ng/mL, and the lower limit of quantification was 0.25 ng/mL for each compound. While the precisely intra-assay and inter-assay variabilities were <9.5 and 7.8 %, respectively; accuracy was determined at the concentrations of 5, 25, 100 ng/mL for all the analytes with the relative standard deviation (%) no more than 15.0 %. Consequently, the validated method could be successfully and precisely applied to the pharmacokinetic study of asperosaponin VI and its metabolites. As a result, the pharmacokinetic parameters of cauloside A, HN saponin F and hederagenin such as T max were obtained at 9.33 ± 2.49, 7.33 ± 0.47 and 12.33 ± 2.36 h, respectively.



Similar content being viewed by others
References
China PCoPR (2010) Pharmacopoeia of People’s Republic of China. China Medical Science and Technology Press, Beijing
Zhang ZJ, Qian YH, Hu HT, Yang J, Yang GD (2003) The herbal medicine Dipsacusasper wall extract reduces the cognitive deficits and overexpression of beta-amyloid protein induced by aluminum exposure. Life Sci 73:2443–2454
Peng LH, Ko CH, Siu SW, Koon CM, Yue GL, Cheng WH, Lau TW, Han QB, Ng KM, Fung KP, Lau CB, Leung PC (2010) In vitro and in vivo assessment of a herbal formula used topically for bone fracture treatment. J Ethnopharmacol 131:282–289
Yang ZG, Ding K, Xu G, Shen Y, Meng ZG, Yang SC (2012) Chemical constituents of Dipsacusasper. J Chin Med Mater 35:1789–1792
Jung KY, Son KH, Do JC (1993) Triterpenoid from the roots of Dipsacusasper. Arch Pharm Res 16:32–35
Li C, Liu Z, Tian J, Li G, Jiang W, Zhang G, Chen F, Lin P, Ye Z (2010) Protective roles of Asperosaponin VI, a triterpene saponin isolated from Dipsacusasper wall on acute myocardial infarction in rats. Eur J Pharmacol 627:235–241
Li C, Tian J, Li G, Jiang W, Xing Y, Hou J, Zhu H, Xu H, Zhang G, Liu Z, Ye Z (2010) Asperosaponin VI protects cardiac myocytes from hypoxia-induced apoptosis via activation of the PI3K/Akt and CREB pathways. Eur J Pharmacol 649:100–107
Jeong SI, Zhou B, Bae JB, Kim NS, Kim SG, Kwon J, Kim DK, Shin TY, Jeon H, Lim JP, Kim H, Kim HK, Oh CH (2008) Apoptosis-inducing effect of akebia saponin D from the roots of Dipsacusasper wall in U937 cells. Arch Pharm Res 31:1399–1400
Zhou YQ, Yang ZL, Xu L, Li P, Hu YZ (2009) Akebia saponin D, a saponin component from Dipsacusasper wall, protects PC 12 cells against amyloid-beta induced cytotoxicity. Cell Biol Int 33:1102–1110
Anisimov MM, Prokofieva NG, Strigina LI, Chetyrina NS, Aladina NG, Elyakov GB (1977) Cells from rat marrow: sensitivity to effects of certain pentacyclic triterpenoid. Biochem Pharmacol 26:2113–2116
Desbene S, Hanquet B, Shoyama Y, Wagner H, Lacaille-Dubois MA (1999) Biologically active triterpene saponins from callus tissue of Polygala amarella. J Nat Prod 62:923–926
Kitanaka S, Yasuda I, Kashiwada Y, Hu CQ (1995) Antitumor agents, 162. Cell-based assays for identifying novel DNA topoisomerase inhibitors: studies on the constituents of Fatsia japonica. J Nat Prod 58:1647–1654
Lee SM, Park JG, Lee YH, Lee CG, Min BS, Kim JH, Lee HK (2004) Anti-complementary activity of triterpenoid from fruits of Zizyphusjujuba. Biol Pharm Bull 27:1883–1886
Oh SR, Jung KY, Son KH, Park SH, Lee IS, Ahn KS, Lee HK (1999) In vitro anticomplementary activity of hederagenin saponins isolated from roots of Dipsacusasper. Arch Pharm Res 22:317–319
Kim SY, Son KH, Chang HW, Kang SS, Kim HP (1999) Inhibition of mouse ear edema by steroidal and triterpenoid saponins. Arch Pharm Res 22:313–316
Takagi K, Park EH, Kato H (1980) Anti-inflammatory activities of hederagenin and crude saponin isolated from Sapindus mukorossi Gaertn. Chem Pharm Bull (Tokyo) 28:1183–1188
Li K, Yang XL, Zhang CF, Yang ZL (2009) Metabolites of Asperosaponin VI in Rats’ Feces by HPLC-MSn. Chin J Nat Med 6:440–443
Zhu H, Ding L, Shakya S, Qi X, Hu L, Yang X, Yang Z (2011) Simultaneous determination of asperosaponin VI and its active metabolite hederagenin in rat plasma by liquid chromatography-tandem mass spectrometry with positive/negative ion-switching electrospray ionization and its application in pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 879:3407–3414
Shakya S, Zhu H, Ding L, Du XL, Qi XM, Yang XL, Yang ZL (2012) Determination of asperosaponin VI in dog plasma by high-performance liquid chromatography-tandem mass spectrometry and its application to a pilot pharmacokinetic study. Biomed Chromatogr 26:109–114
Wu S, Liu EW, Zhang Y, Han LF, Liu H (2010) Studies on chemical constituents in dipsacusasperoides. J Tianjin Tradit Chin Med Univ 3:147–150
Li K, Ding L, Yang ZL, Liu EH, Qi LW, Li P, Hu YZ (2010) Determination of asperosaponin VI in rat plasma by HPLC-ESI-MS and its application to preliminary pharmacokinetic studies. Biomed Chromatogr 24:550–555
Mach J, Huizer-Pajkos A, Cogger VC, McKenzie C, Le Couteur DG, Jones BE, de Cabo R, Hilmer SN (2013) The effect of aging on acetaminophen pharmacokinetics, toxicity and Nrf2 in Fischer 344 rats. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glt095
Acknowledgments
This research was supported by Program for Important Drug Development of MOST, China (2012ZX09304007), 2013ZX09401-004-002, and 2011ZX09307-002-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, EW., Wang, JL., Han, LF. et al. Pharmacokinetics study of asperosaponin VI and its metabolites cauloside A, HN saponin F and hederagenin. J Nat Med 68, 488–497 (2014). https://doi.org/10.1007/s11418-014-0821-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-014-0821-4