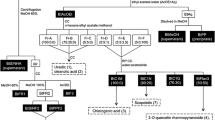
Abstract
Not only neuronal death but also neuritic atrophy and synaptic loss underlie the pathogenesis of Alzheimer’s disease as direct causes of the memory deficit. Extracts of Siberian ginseng (the rhizome of Eleutherococcus senticosus) were shown to have protective effects on the regeneration of neurites and the reconstruction of synapses in rat cultured cortical neurons damaged by amyloid β (Aβ)(25–35), and eleutheroside B was one of the active constituents. In this study, a comprehensive evaluation of constituents was conducted to explore active components from Siberian ginseng which can protect against neuritic atrophy induced by Aβ(25–35) in cultured rat cortical neurons. The ethyl acetate, n-butanol and water fractions from the methanol extract of Siberian ginseng showed protective effects against Aβ-induced neuritic atrophy. Twelve compounds were isolated from the active fractions and identified. Among them, eleutheroside B, eleutheroside E and isofraxidin showed obvious protective effects against Aβ(25–35)-induced atrophies of axons and dendrites at 1 and 10 μM.



Similar content being viewed by others
References
Deyama T, Nishibe D, Nakazawa Y (2001) Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin 22:1057–1070
Gaffeny BT, Hügel HM, Rich PA (2001) Panax ginseng and Eleutherococcus senticosus may exaggerate an already existing biphasic response to stress via inhibition of enzymes which limit the binding of stress hormones to their receptors. Med Hypotheses 56:567–572
Fujikawa T, Miguchi S, Kanada N, Nakai N, Ogata M, Suzuki I, Nakashima K (2005) Acanthopanax senticosus Harms as a prophylactic for MPTP-induced Parkinson’s disease in rats. J Ethnopharmacol 97:375–381
Tohda C, Ichimura M, Bai YJ, Tanaka K, Zhu S, Komatsu K (2008) Inhibitory effects of Eleutherococcus senticosus extracts on amyloid β(25–35)-induced neuritic atrophy and synaptic loss. J Pharmacol Sci 107:329–339
Seltzer B (2007) Donepezil: an update. Expert Opin Pharmacother 8:1011–1023
Dickson TC, Vichkers JC (2001) The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience 105:99–107
Kuboyama T, Tohda C, Komatsu K (2006) Withanoside IV and its active metabolite, sominone, attenuate Aβ(25–35)-induced neurodegeneration. Eur J Neurosci 23:1417–1427
Evans NA, Facci L, Owen DE (2008) Abeta(1–42) reduces synapse number and inhibits neurite outgrowth in primary cortical and hippocampal neurons: a quantitative analysis. J Neurosci Methods 175:96–103
Kurkin VA (2003) Phenylpropanoids from medicinal plants: distribution, classification, structural analysis, and biological activity. Chem Nat Compd 39:123–153
Kuboyama T, Tohda C, Komatsu K (2005) Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol 144:961–971
Kiem PV, Minh CV, Dat NT, Cai XF, Lee JJ, Kim YH (2003) Two new phenylpropanoid glycosides from the stem bark of Acanthopanax trifoliatus. Arch Pharm Res 26:1014–1017
Gaffney BT, Hugel HM, Rich PA (2004) The chromatographic co-elution of dihydrodehydrodiconiferyl alcohol monopyranose with eleutheroside E in Eleutherococcus senticosus: implications for eleutheroside E assays. Phytochem Anal 15:231–234
Nishibe S, Kinoshita H, Takeda H, Okano G (1990) Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stressed rats. Chem Pharm Bull 38:1763–1765
Ryu J, Son D, Kang J, Kim HS, Kim BK, Sanghyun L (2004) A benzenoid from the stem of Acanthopanax senticosus. Arch Pharm Res 27:912–914
Kim DK, Lim JP, Kim JW, Park HW, Eun JS (2005) Antitumor and anti-inflammatory constituents from Celtis sinensis. Arch Pharm Res 28:39–43
Tsukamoto H, Hisada S, Nishibe S (1985) Coumarins from bark of Fraxinus japonica and F. mandshurica var. japonica. Chem Pharm Bull 33:4069–4073
Damrong K, Juraithip W, Wanchai DE (2008) Biosynthesis of β-sitosterol and stigmasterol proceeds exclusively via the mevalonate pathway in cell suspension cultures of Croton stellatopilosus. Tetrahedron Lett 49:4067–4072
Whitehouse PJ, Price DL, Clark AW, Coyle JT, Delong MR (1981) Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 10:122–126
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology 50:136–145
Ogura H, Kosasa T, Yamanishi Y (2000) Donepezil, a centrally acting acetylcholinesterase inhibitor, alleviates learning deficits in hypocholinergic models in rats. Methods Find Exp Clin Pharmacol 22:89–95
Bobinski M, Wegiel J, Tarnawski M, Bobinski M, Reisberg B, Leon MJ (1997) Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer’s disease. J Neuropathol Exp Neurol 56:414–420
Tohda C, Matsumoto N, Zou K, Meselhy R, Komatsu K (2004) Aβ (25–35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, a metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology 29:860–868
Thoda C, Tamura T, Matsuyama S, Komatsu K (2006) Promotion of axonal maturation and inhibition of dementia by Astragalus mongholicus. Br J Pharmacol 149:532–541
Patrick JD, Lamprecht JH (1999) Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev 4:170–177
Boulic PJD, Etsebeth S, Liebengerg RW (1996) Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int J Immunopharmacol 18:693–700
Sun H, Lv H, Zhang Y-M, Wang X-J, Bi K-S, Cao H-X (2007) Pharmacokinetics of isofraxidin in rat plasma after oral administration of the extract of Acanthopanax senticosus using HPLC with solid phase extraction method. Chem Pharm Bull 55:1291–1295
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B), No. 21406004 in 2009–2010, from the Japan Society for the Promotion of Science and by Expansion Program, Regional Innovation Cluster Program, Global Type (II), “Hokuriku Innovation Cluster for Health Science” from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, Y., Tohda, C., Zhu, S. et al. Active components from Siberian ginseng (Eleutherococcus senticosus) for protection of amyloid β(25–35)-induced neuritic atrophy in cultured rat cortical neurons. J Nat Med 65, 417–423 (2011). https://doi.org/10.1007/s11418-011-0509-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-011-0509-y