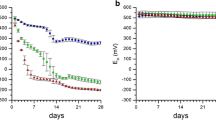
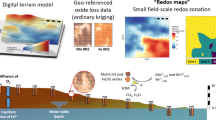
Abstract
Purpose
When studying redox conditions in soils with manganese (Mn) and iron (Fe) oxide-coated redox bars, we observed the formation of Fe oxides along the Mn oxide coating and assumed sorption of other elements from soil solution to oxide surface. The objective of this study was to investigate the formation of Fe oxides along Mn redox bars and to analyze element sorption from soil solution to either Mn or Fe oxide along redox bar coatings.
Materials and methods
We protruded Mn redox bars into solutions with defined Fe2+ concentrations and removed the bars at distinct time intervals. The Mn oxide coating and potential Fe oxides were extracted using dithionite-citrate-bicarbonate (DCB). To investigate in situ element sorption behavior, we used previously field-installed redox bars, protruding these Mn redox bars into acidified hydroxylamine hydrochloride (AAH) to selectively extract Mn oxide and afterwards into DCB to dissolve the remaining Fe oxide coating. This two-step extraction procedure enabled the differentiation of elements bonded to either Mn or Fe oxide. Additionally, we analyzed the redox bar coatings at a very small scale (<1 mm2) via energy-dispersive x-ray spectroscopy (EDX).
Results and discussion
Iron oxides precipitated along the Mn oxide coating at low concentrations of 0.05 mg Fe2+ L−1, but did not trigger a color change. Although a change in color did occur instantaneously at 500 mg Fe2+ L−1, it is expected that Fe2+ concentrations are significantly lower under field conditions because ferrous Fe auto-oxidized within the artificial setup. Whereas Mn oxide sorbed cationic elements from the soil solution in the order Cu > Pb > Zn, Fe oxide preferentially sorbed oxyanions with As > P > Mo > V, respectively. “Field”-Fe oxides precipitating along the Mn redox bars sorbed elevated levels of As and P compared with the action of synthesized “lab”-Fe oxides along Fe redox bars, a finding which we attribute to short-range-ordered Fe phases with elevated sorption capacity.
Conclusions
Besides providing information regarding the monitoring of soil redox status, the developed sequential two-step extraction procedure enables the differentiation of the selective sorption of elements in the soil solution to the coating of Mn and Fe redox bars. The collection of Fe oxides formed naturally along the Mn redox bar coatings further enables the investigation of temporally and spatially diverse Fe oxide-forming processes.






Similar content being viewed by others
References
Abd-Elfattah ALY, Wada K (1981) Adsorption of lead, copper, zinc, cobalt, and cadmium by soils that differ in cation-exchange materials. J Soil Sci 32:271–283
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals, 2nd edn. Springer, Heidelberg
Ajouyed O, Hurel C, Ammari M, Allal LB, Marmier N (2010) Sorption of Cr(VI) onto natural iron and aluminum (oxy)hydroxides: effects of pH, ionic strength and initial concentration. J Hazard Mater 174:616–622
Arai Y (2008) Spectroscopic evidence for Ni(II) surface speciation at the iron oxyhydroxides−water interface. Environ Sci Technol 42:1151–1156
Atta SK, Mohammed SA, Van Cleemput O, Zayed A (1996) Transformations of iron and manganese under controlled EH, EH-pH conditions and addition of organic matter. Soil Technol 9:223–237
Barling J, Anbar AD (2004) Molybdenum isotope fractionation during adsorption by manganese oxides. Earth Planet Sci Lett 217:315–329
Borggaard OK (1983) Effects of surface area and mineralogy of iron oxides on their surface charge and anion-adsorption properties. Clay Clay Miner 31:230–232
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18
Brümmer GW, Gerth J, Tiller KG (1988) Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite. I. adsorption and diffusion of metals. J Soil Sci 39:37–52
Castenson KL, Rabenhorst MC (2006) Indicator of reduction in soil (IRIS): evaluation of a new approach for assessing reduced conditions in soil. Soil Sci Soc Am J 70:1222–1226
Chao TT (1972) Selective dissolution of manganese oxides from soils and sediments with acidified hydroxylamine hydrochloride. Soil Sci Soc Am J 36:764–768
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurences, and uses, 2nd edn. Wiley-VCH, Weinheim
Della Puppa L, Komárek M, Bordas F, Bollinger JC, Joussein E (2013) Adsorption of copper, cadmium, lead and zinc onto a synthetic manganese oxide. J Colloid Interface Sci 399:99–106
Dorau K, Mansfeldt T (2015) Manganese-oxide-coated redox bars as an indicator of reducing conditions in soils. J Environ Qual 44:696–703
Dorau K, Eickmeier M, Mansfeldt T (2015) Comparison of manganese and iron oxide-coated redox bars for characterization of the redox status in wetland soils. WETLANDS revised version under review
Feng XH, Zhai LM, Tan WF, Liu F, He JZ (2007) Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ Pollut 147:366–373
Giménez J, Martínez M, de Pablo J, Rovira M, Duro L (2007) Arsenic sorption onto natural hematite, magnetite, and goethite. J Hazard Mater 141:575–580
Goldberg T, Archer C, Vance D, Poulton SW (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr)oxides. Geochim Cosmochim Acta 73:6502–6516
Golden DC, Dixon JB, Chen CC (1986) Ion exchange, thermal transformations, and oxidizing properties of birnessite. Clay Clay Miner 34:511–520
Gotoh S, Patrick WH (1972) Transformation of manganese in a waterlogged soil as affected by redox potential and pH. Soil Sci Soc Am Proc 36:738–742
Gotoh S, Patrick WH (1974) Transformation of iron in a waterlogged soil as influenced by redox potential and pH. Soil Sci Soc Am Proc 38:66–71
Han R, Zou W, Li H, Li Y, Shi J (2006) Copper(II) and lead(II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite. J Hazard Mater 137:934–942
Hindersmann I, Mansfeldt T (2014) Trace element solubility in a multimetal-contaminated soil as affected by redox conditions. Water Air Soil Pollut 225:1–20
Hooda PS (2010) Trace elements in soils, 1st edn. Wiley, West Sussex
Jenkinson BJ, Franzmeier DP (2006) Development and evaluation of iron-coated tubes that indicate reduction in soils. Soil Sci Soc Am J 70:183–191
Jolivet JP, Chanéac C, Tronc E (2004) Iron oxide chemistry. From molecular clusters to extended solid networks. Chem Commun 5:481–483
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides—a review. Environ Pollut 172:9–22
Kosman DJ (2013) Iron metabolism in aerobes: managing ferric iron hydrolysis and ferrous iron autoxidation. Coord Chem Rev 257:210–217
Manning BA, Goldberg S (1997) Arsenic(III) and arsenic(V) adsorption on three california soils. Soil Sci 162:886–895
Mansfeldt T, Overesch M (2013) Arsenic mobility and speciation in a Gleysol with petrogleyic properties: a field and laboratory approach. J Environ Qual 42:1130–1141
Mansfeldt T, Schuth S, Häusler W, Wagner F, Kaufhold S, Overesch M (2012) Iron oxide mineralogy and stable iron isotope composition in a Gleysol with petrogleyic properties. J Soils Sediments 12:97–114
Matern K, Mansfeldt T (2015) Molybdate adsorption by birnessite. Appl Clay Sci 108:78–83
McKenzie R (1980) The adsorption of lead and other heavy metals on oxides of manganese and iron. Aust J Soil Res 18:61–73
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clay Clay Miner 7:317–327
Morse JW, Luther GW (1999) Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochim Cosmochim Acta 63:3373–3378
Nesbitt HW, Canning GW, Bancroft GM (1998) XPS study of reductive dissolution of 7Å-birnessite by H3AsO3, with constraints on reaction mechanism. Geochim Cosmochim Acta 62:2097–2110
Ottow JCG (2011) Microbiology of soils (In German), 1st edn. Springer, Heidelberg
Peacock CL, Sherman DM (2004) Vanadium(V) adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: a surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim Cosmochim Acta 68:1723–1733
Peretyazhko T, Sposito G (2005) Iron(III) reduction and phosphorous solubilization in humid tropical forest soils. Geochim Cosmochim Acta 69:3643–3652
Rabenhorst MC, Ming DW, Morris RV, Golden DC (2008) Synthesized iron oxides used as a tool for documenting reducing conditions in soils. Soil Sci 173:417–423
Rai D, Eary LE, Zachara JM (1989) Environmental chemistry of chromium. Sci Total Environ 86:15–23
Raven KP, Jain A, Loeppert RH (1998) Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environ Sci Technol 32:344–349
Rennert T, Mueller CW, Mansfeldt T, Lugmeier J (2013) Collecting in situ precipitated iron oxides in their natural soil environment. J Plant Nutr Soil Sci 176:497–499
Roberts DR, Scheinost AC, Sparks DL (2002) Zinc speciation in a smelter-contaminated soil profile using bulk and microspectroscopic techniques. Environ Sci Technol 36:1742–1750
Scheinost AC, Kretzschmar R, Pfister S, Roberts DR (2002) Combining selective sequential extractions, x-ray absorption spectroscopy, and principal component analysis for quantitative zinc speciation in soil. Environ Sci Technol 36:5021–5028
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Strauss R, Brümmer GW, Barrow NJ (1997) Effects of crystallinity of goethite: II. Rates of sorption and desorption of phosphate. Euro J Soil Sci 48:101–114
Takematsu N, Sato Y, Okabe S, Nakayama E (1985) The partition of vanadium and molybdenum between manganese oxides and sea water. Geochim Cosmochim Acta 49:2395–2399
Thompson A, Chadwick OA, Rancourt DG, Chorover J (2006) Iron-oxide crystallinity increases during soil redox oscillations. Geochim Cosmochim Acta 701710–1727
Wang X, Liu F, Tan W, Feng X, Koopal LK (2013a) Transformation of hydroxycarbonate green rust into crystalline iron (hydr)oxides: influences of reaction conditions and underlying mechanisms. Chem Geol 351:57–65
Wang X, Liu F, Tan W, Li W, Feng X, Sparks DL (2013b) Characteristics of phosphate adsorption-desorption onto ferrihydrite: comparison with well-crystalline Fe (hydr)oxides. Soil Sci 178:1–11
Yin H, Feng X, Tan W, Koopal LK, Hu T, Zhu M, Liu F (2015) Structure and properties of vanadium(V)-doped hexagonal turbostratic birnessite and its enhanced scavenging of Pb2+ from solutions. J Hazard Mater 288:80–88
Zachara JM, Girvin DC, Schmidt RL, Resch CT (1987) Chromate adsorption on amorphous iron oxyhydroxide in the presence of major groundwater ions. Environ Sci Technol 21:589–594
Acknowledgments
This study was financially supported by Verein der Freunde und Förderer der Universität zu Köln. We would like to thank Karin Greef (University of Cologne) and Gerd Welp and Addi Kiener (University of Bonn) for analyzing the extracts, and Ruth Bruker and Stefan Roitsch (University of Cologne) for EDX analysis. Additionally, we are grateful to the Duke of Croy and Thomas Seine, who enabled the field measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Rights and permissions
About this article
Cite this article
Dorau, K., Mansfeldt, T. Manganese and iron oxide-coated redox bars as a tool to in situ study the element sorption in wet soils. J Soils Sediments 16, 976–986 (2016). https://doi.org/10.1007/s11368-015-1300-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1300-6