Abstract
Purpose
A Product Category Rules (PCR) document specifies the quantification method and communication format of environmental impacts of a product category. To ensure neutrality and credibility of quantitative environmental information, the development of PCR documents is defined in ISO 14025. Hence, the rules are preconditions for comparative considerations and modular application of information entities and Environmental Product Declarations (EPD). However, with the growing number of EPD programs, the producers, purchasers, and consumers feel increasingly alienated in relation to the validity and legitimacy of the environmental information presented by EPDs. This results in a need for enhanced transparency of PCR development and EPD program compatibility. This article offers navigational assistance in this respect.
Methods
To identify harmonization potential, we compare PCR development regarding two aspects: quantitatively by mapping existing PCR and EPD documents, and qualitatively by comparing existing institutional structures with normative guidance. Information was gathered through an internet search and through direct correspondence with program operators.
Results and discussion
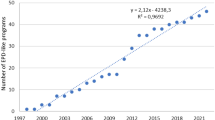
We identified 27 programs, 556 PCR documents, and 3614 EPD declarations (May 2013). There were significant differences in activity level between programs and sectors. Furthermore, the institutional structures differ widely from each other and from normative guidance on PCR development.
Conclusions
This first global PCR register guides practitioners in the search for PCR documents. The analysis of program institutional structures for PCR development and EPD verification indicates the involvement of different stakeholders on Type III environmental declarations. Regarding PCR compatibility and newly released guidance documents from the European Commission and the PCR Guidance Development Initiative, we recommend that operators (1) settle on a common (sector) categorization system, (2) implement a Stakeholder Identification Worksheet, (3) consider the mandatory involvement of consumer and environmental interests in the PCR review panel, (4) require PCR reviewers to declare potential conflicts of interest, and (5) consider installing mandatory third party verification of declarations for any external use.



Similar content being viewed by others
References
ACLCA [American Center for Life Cycle Assessment] (n.d.) Guidance for Product Category Rule Program Developers. URL: http://www.lcacenter.org/Data/Sites/1/SharedFiles/committeedocuments/pcrcommittee/guidanceforpcrdevelopers.pdf (07.11.2012)
Crespi JM, Marette S (2005) Eco-labelling economics: is public involvement necessary? In: Krarup S, Clifford SR (eds) Environment, information and consumer behavior. Edward Elgar Publishing Ltd, Cheltenham
Dahlbo H et al (2012) Comparison of different normalised LCIA results and their feasibility in communication. Int J Life Cycle Assess. Online publication 28.09.2012. http://link.springer.com/article/10.1007%2Fs11367-012-0498-4 (03.11.2012)
Defra [Department for Environmental, Food and Rural Affairs] (2007) Changing behaviour through policy making. http://archive.defra.gov.uk/sustainable/government/documents/change-behaviour-model.pdf (09.07.2013)
Del Borghi A (2012) LCA and communication: Environmental Product Declaration. Int J Life Cycle Assess. Online publication 27.09.2012. http://download.springer.com/static/pdf/139/art%253A10.1007%252Fs11367-012-0513-9.pdf?auth66=1352123647_13feb6cbec220cd873dd4fc74d82a494&ext=.pdf (05.11.2012)
Del Borghi A et al (2008) Development of PCR for WWTP based on a case study. Int J Life Cycle Assess 13:512–521
EC [European Commission] (2011) Roadmap to a Resource Efficient Europe. Communication from the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2011:0571:FIN:EN:PDF (10.11.2012)
EC [European Commission] (2013) Commission Recommendation of 9 April 2013 on the use of common methods to measure and communicate the life cycle environmental performance of products and organisations. Annex II. Product Environmental Footprint (PEF) Guide. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:124:FULL:EN:PDF (19.06.2013)
EC-JRC-IES [European Commission – Joint Research Centre – Institute for Environment and Sustainability] (2011): Analysis of Existing Environmental Footprint Methodologies for Products and Organizations: Recommendations, Rationale, and Alignment. Published by the European Commission, DG Environment. http://ec.europa.eu/environment/eussd/pdf/Deliverable.pdf (05.11.2012)
EN 15804 (2012) Sustainability of construction works - Environmental product declarations - Core rules for the product category of construction products. http://esearch.cen.eu/esearch/Details.aspx?id=15457000 (10.12.2013)
Fet AM et al (2009) Product category rules and environmental product declarations as tools to promote sustainable products: experiences from a case study of furniture production. Clean Technol Environ Policy 11:201–207
Hauschild MZ et al (2013) Identifying best existing practice for characterization modeling in life cycle impact assessment. J Clean Prod 18:683–697
Ingwersen W, Stevenson MJ (2012) Can we compare the environmental performance of this product to that one? An update on the development of product category rules and future challenges toward alignment. J Clean Prod 24:102–108
Ingwersen W, Subramanian V (eds) (2013) Guidance for Product Category Rules Development. http://www.pcrguidance.org/ (30.08.2013)
ISO 14025 (2006) Environmental labels and declarations – Type III environmental declarations – Principles and procedures. http://www.iso.org/iso/catalogue_detail?csnumber=38131 (19.06.2013)
ISO/TS 14067 (2013) Greenhouse gases – Carbon footprint of products – Requirements and guidelines for quantification and communication. http://www.iso.org/iso/catalogue_detail?csnumber=59521 (19.06.2013)
Leire C, Thidell Å (2005) Product-related environmental information to guide consumer purchases – a review and analysis of research on perceptions, understanding and use among Nordic consumers. J Clean Prod 13:1061–1071
Magerøy M (2011) The communication of environmental impacts through environmental product declarations. Master Thesis, Norwegian University of Science and Technology, Trondheim. http://www.ntnu.edu/c/document_library/get_file?uuid=692dae26-0294-451c-a0f7-e060b423c675&groupId=163835 (02.11.2012)
Modahl IS et al (2013) Comparison of two versions of an EPD, using generic and specific data for the foreground system, and some methodological implications. Int J Life Cycle Assess 18:241–251
Nakaniwa C (2003) Harmonization of Type III Environmental Declaration Programs and the Application Toward Sustainable Consumption. The Global Type III Environmental Product Declarations Network (GEDnet). Part of The First International Workshop on Sustainable Consumption Report. http://www.aist-riss.jp/old/lca/cie/activity/project/sc/report/program030319.html (22.06.2013)
Schau EM, Fet AM (2008) LCA studies of food products as background for environmental product declarations. Int J Life Cycle Assess 3:255–264
Schmincke E (2011) The European standard FprEN 15804 for EPD in the construction sector and the application of the modularity principle. Paper at LCM 28.-31.08.2011 in Berlin. http://www.lcm2011.org/papers.html (10.12.2013)
Schmincke E, Grahl B (2006) Umwelteigenschaften von Produkten. Die Rolle der Okobilanz in ISO Typ III Umweltdeklarationen. Umweltwissenschaften und Schadstoff-Forschung 3:185–192
Subramanian V (2013) Integration of Product Category Rules from North America into the Global PCR Database. Report by PRé North America Inc. for GEDnet. http://media.gednet.org/2013/05/2012_07_30_Report_PCR-in-the-US-Canada_Deliverable.pdf (02.07.2013)
Subramanian V et al (2012) Comparing product category rules from different programs: learned outcomes towards global alignment. Int J Life Cycle Assess 7:892–903
Wardenaar T et al (2012) Differences between LCA for analysis and LCA for policy: a case study on the consequences of allocation choices in bio-energy policies. Int J Life Cycle Assess 17:1059–1067
Acknowledgments
This work was funded by the Society for Soil and Water Protection (Gesellschaft für Boden- und Gewässerschutz e.V., Wettenberg, Germany) and the Institute for Applied Ecology (Öko-Institut e.V., Freiburg, Germany). The views expressed in this article are not necessarily endorsed by these institutions. We especially thank Carl-Otto Gensch at the Institute for Applied Ecology for cooperation and support and the reviewers for their critical comments and proposals for improvement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Birgit Grahl
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 119 kb)
Rights and permissions
About this article
Cite this article
Hunsager, E.A., Bach, M. & Breuer, L. An institutional analysis of EPD programs and a global PCR registry. Int J Life Cycle Assess 19, 786–795 (2014). https://doi.org/10.1007/s11367-014-0711-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-014-0711-8