Abstract
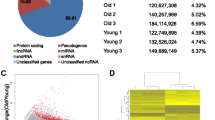
The ability of the liver to regenerate and adjust its size after two/third partial hepatectomy (PH) is impaired in old rodents and humans. Here, we investigated by microarray analysis the expression pattern of hepatic genes in young and old untreated mice and the differences in gene expression profile following PH. Of the 10,237 messenger RNAs that had detectable expression, only 108 displayed a greater than 2-fold modification in gene expression levels between the two groups. These genes were involved in inflammatory and immune response, xenobiotics, and lipid and glucose metabolism. To identify the genes responsible for the different regenerative response, 10-week and 18-month-old mice subjected to PH were sacrificed at different time intervals after surgery. The results showed that 2463 transcripts had significantly different expression post PH between the two groups. However, in spite of impaired liver regeneration in old mice, cell cycle genes were similarly modified in both groups, the only exception being cyclin D1 gene which was up-regulated soon after PH in young mice, but mostly down-regulated in aged animals. Surprisingly, while in young hepatectomized mice, Yap messenger RNA (mRNA) expression was not significantly enhanced and protein expression essentially reflected the progression into cell cycle, its mRNA and protein levels were robustly increased in the liver of aged animals. Furthermore, a significant change of the age-related expression of the size regulator Yes-associated protein (YAP) was observed. Unexpectedly, while in young hepatectomized mice, Yap mRNA expression was not significantly enhanced and protein expression essentially reflected the progression into cell cycle, its mRNA and protein levels were robustly increased in the liver of aged animals. Moreover, when PH was performed on mitogen-induced enlarged livers, the earlier restoration of the original liver mass compared to animals subjected to PH only led to YAP down-regulation concomitantly with cyclin D1 up-regulation. Our data suggest that YAP activation is a size-dependent homeostatic mechanism that does not necessarily reflect cell cycle progression.





Similar content being viewed by others
Abbreviations
- AP-1:
-
Activating protein-1
- BrdU:
-
Bromodeoxyuridine
- Cebp:
-
CCAAT/enhancer-binding protein (C/EBP)
- egr 1:
-
Early growth response-1
- Foxm1:
-
Forkhead box M1
- HGF:
-
Hepatocyte growth factor
- Keap1:
-
Kelch-like ECH-associated protein 1
- IL-6:
-
Interleukin-6
- IHC:
-
Immunohistochemistry
- NF-κB:
-
Nuclear factor-κB
- Nqo2:
-
NAD(P)H dehydrogenase quinone 2
- NRF2:
-
Nuclear factor (erythroid-derived 2)-like 2
- PCNA:
-
Proliferating cell nuclear antigen
- PH:
-
Partial hepatectomy
- qRT-PCR:
-
Quantitative reverse transcriptase polymerase chain reaction
- STAT3:
-
Signal transducer and activator of transcription factor 3
- TAZ:
-
Transcriptional co-activator
- TCPOBOP:
-
1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
- YAP:
-
Yes-associated protein
References
Ballou SP, Lozanski FB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, Ford AB, Kushner I (1996) Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing 25:224–230
Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S (2008) Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J 27:212–223
Bucher NLR, Swaffield MN, Di Troia JF (1964) The influence of age upon the incorporation of thymidine-2-C14 into the DNA of regenerating rat liver. Cancer Res 24:509–512
Burzynski SR (2003) Gene silencing a new theory of aging. Med Hypotheses 60:578–583
Burzynski SR (2005) Aging: gene silencing or gene activation? Med Hypotheses 64:201–208
Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R et al (2007) YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17:2054–2060
Columbano A, Simbula M, Pibiri M, Perra A, Pisanu A, Uccheddu A, Ledda-Columbano GM (2008) Potential utility of xenobiotic mitogens in the context of liver regeneration in the elderly and living-related transplantation. Lab Investig 88:408–415
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383
Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA et al (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130:1120–1133
Fausto N, Laird AD, Webber EM (1995) Role of growth factors and cytokines in hepatic regeneration. Faseb J 9:1527–1536
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Fry MF, Silber J, Loeb LA et al (1984) Delayed and reduced cell replication and diminishing levels of DNA polymerases-α in regenerating liver of aging mice. J Cell Physiol 118:225–232
Gan L, Chitturi S, Farrell GC (2011) Mechanisms and implications of age-related changes in the liver: nonalcoholic fatty liver disease in the elderly. Curr Gerontol Geriatr Res 831536
Grana X, Reddy PE (1995) Cell cycle control in mammalian cells, role of cyclins, cyclin-dependent kinases (CDKs), growth suppressor genes and cyclin dependent kinase inhibitors (CKIs) 11:211–219
Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, Sadri-Vakili G, Vakili K (2014) Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol 307:G196–G204
Higgins GM, Anderson RM (1931) Experimental pathology of the liver. Restoration of the liver of the white rat following partial removal. Arch Pathol 12:186–202
Iakova P, Awad SS, Timchenko N (2003) Aging reduced proliferative capacities of liver by switching pathway of C/EBPα growth arrest. Cell 113:495–506
Köhler UA, Kurinna S, Schwitter D, Marti A, Schäfer M, Hellerbrand C, Speicher T, Werner S (2014) Activated Nrf2 impairs liver regeneration in mice by activation of genes involved in cell-cycle control and apoptosis. Hepatology 60:670–678
Ledda-Columbano GM, Pibiri M, Cossu C, Molotzu F, Locker J, Columbano A (2004) Aging does not reduce the hepatocyte proliferative response of mice to the primary mitogen TCPOBOP. Hepatology 40:981–988
Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276:60–66
Pan D (2010) The hippo signaling pathway in development and cancer. Dev Cell 19:491–505
Praticò D (2002) Lipid peroxidation and the aging process. Sci Aging Knowl Environ (50)re5
Premoli A, Paschetta E, Hvalryg M, Spandre M, Bo S, Durazzo M (2009) Characteristics of liver diseases in the elderly: a review. Minerva Gastroenterol Dietol 55:71–78
Schmucker DL (2005) Age-related changes in liver structure and function: implications for disease? Exp Gerontol 40:650–659
Sheedfar F, Di Biase S, Koonen D, Vinciguerra M (2013) Liver diseases and aging: friends or foes? Aging Cell 12:950–954
Singh P, Goode T, Dean A, Awad SS, Darlington GJ (2011) Elevated interferon gamma signaling contributes to impaired regeneration in the aged liver. J Gerontol A Biol Sci Med Sci 66:944–956
Taub R (1996) Transcriptional control of liver regeneration. Faseb J 10:413–427
Timchenko NA (2009) Aging and liver regeneration. Trends Endocrinol Metab 20(4):171–176
Vranckx R, Savu L, Lambert N, de Conchard GV, Grosse R, Mourey MS, Corman B (1995) Plasma proteins as biomarkers of the aging process. Am J Physiol 268:R536–R548
Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH (2001) Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age related proliferation defects in regenerating liver. PNAS 20:11468–11473
Wang C, Zhang L, He Q, Feng X, Zhu J, Xu Z, Wang X, Chen F, Li X, Dong J (2012) Differences in Yes-associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Mol Med Rep 5:410–414
Yamada Y, Kirillova I, Peschon JJ, Fausto N (1997) Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 94(4):1441–1446
Zhu C, Ikemoto T, Utsunomiya T, Yamada S, Morine Y, Imura S, Arakawa Y, Takasu C, Ishikawa D, Shimada M (2014) Senescence-related genes possibly responsible for poor liver regeneration after hepatectomy in elderly patients. J Gastroenterol Hepatol 29:1102–1108
Acknowledgments
Work supported by Italian Ministry of Health (Grant Young Researcher GR-2011-02350476 to M.R. and GR-2011-02347781to G.N.), Italian Ministry for Education, University and Research (Grants PRIN 2010LC747T), National Research Council Flagship Project Interomics, University of Salerno (FARB 2014), and Fondazione Banco di Sardegna to A.P.; G.N. is supported by a ‘Mario e Valeria Rindi’ fellowship of the Italian Foundation for Cancer Research. M.A.K. is a fellow of the Associazione Italiana Ricerca sul Cancro.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Monica Pibiri and Pia Sulas contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(XLS 37 kb)
Supplementary Table 2
(XLS 123 kb)
Supplementary Table 3
(XLS 646 kb)
Supplementary Table 4
(XLS 58 kb)
Supplementary Table 5
(XLS 43 kb)
ESM 6
(DOC 27 kb)
About this article
Cite this article
Pibiri, M., Sulas, P., Leoni, V.P. et al. Global gene expression profile of normal and regenerating liver in young and old mice. AGE 37, 59 (2015). https://doi.org/10.1007/s11357-015-9796-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-015-9796-7