Abstract
The lens is an ideal model system for the study of macromolecular aging and its consequences for cellular function, since there is no turnover of lens fibre cells. To examine biochemical processes that take place in the lens and that may also occur in other long-lived cells, membranes were isolated from defined regions of human lenses that are synthesised at different times during life, and assayed for the presence of tightly bound cytosolic proteins using quantitative iTRAQ proteomics technology. A majority of lens beta crystallins and all gamma crystallins became increasingly membrane bound with age, however, the chaperone proteins alpha A and alpha B crystallin, as well as the thermally-stable protein, βB2 crystallin, did not. Other proteins such as brain-associated signal protein 1 and paralemmin 1 became less tightly bound in the older regions of the lens. It is evident that protein–membrane interactions change significantly with age. Selected proteins that were formerly cytosolic become increasingly tightly bound to cell membranes with age and are not removed even by treatment with 7 M urea. It is likely that such processes reflect polypeptide denaturation over time and the untoward binding of proteins to membranes may alter membrane properties and contribute to impairment of communication between older cells.




Similar content being viewed by others
References
Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM (2003) Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Investig Ophthalmol Vis Sci 44:5287–5292
Aisenbrey C, Borowik T, Byström R, Bokvist M, Lindström F, Misiak H, Sani M-A, Gröbner G (2008) How is protein aggregation in amyloidogenic diseases modulated by biological membranes? Eur Biophys J 37:247–255
Bagchi M, Katar M, Lo WK, Maisel H (2003) Paralemnin of the lens. J Cell Biochem 89:917–921
Bagchi M, Kousis S, Maisel H (2008) BASP1 in the lens. J Cell Biochem 105:699–702
Ball L, Garland D, Crouch R, Schey K (2004) Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurence. Biochemistry 43:9856–9865
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102
Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J (2006) Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci 103:12564–12568
Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A (2004) Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol 86:407–485
Borchman D, Byrdwell WC, Yappert MC (1994) Regional and age-dependent differences in the phospholipid composition of human lens membranes. Investig Ophthalmol Vis Sci 35:3938–3942
Bron AJ, Vrensenb GFJM, Koretzc J, Marainid G, Hardinga JJ (2000) The Ageing lens. Ophthalmologica 214:86–104
Bulteau AL, Petropoulos I, Friguet B (2000) Age-related alterations of proteasome structure and function in aging epidermis. Exp Gerontol 35:767–777
Castellini M, Wolf L, Chauhan B, Galileo D, Kilimann M, Cvekl A, Duncan M (2005) Palm is expressed in both developing and adult mouse lens and retina. BMC Ophthalmol 5:14
Chandrasekher G, Cenedella RJ (1995) Protein associated with human lens ‘native’ membrane during aging and cataract formation. Exp Eye Res 60:707–717
Derham BK, Harding JJ (1997) Effect of aging on the chaperone-like function of human alpha-crystallin assessed by three methods. Biochem J 328(Pt3):763–768
Dobson CM (2001) The structural basis of protein folding and its links with human disease. Philos Trans R Soc Lond B Biol Sci 356:133–145
Fagerholm PP, Philipson BT, Lindstrom B (1981) Normal human lens—the distribution of protein. Exp Eye Res 33:615–620
Fan X, Zhang J, Theves M, Strauch C, Nemet I, Liu X, Qian J, Giblin FJ, Monnier VM (2009) Mechanism of lysine oxidation in human lens crystallins during aging and in diabetes. J Biol Chem 284:34618–34627, %R 34610.31074/jbc.M34109.032094
Feng J, Smith DL, Smith JB (2000) Human lens beta-crystallin solubility. J Biol Chem 275:11585–11590
Fields RD, Stevens-Graham B (2002) New insights into neuron-glia communication. Science 298:556–562
Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS (1996) Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci 93:4765
Friedrich MG, Truscott RJW (2009) Membrane association of proteins in the aging human lens: profound changes take place in the 5th decade of life. Investig Ophthalmol Vis Sci 50:4786–4793
Gao J, Yin D, Yao Y, Williams TD, Squier TC (1998) Progressive decline in the ability of calmodulin isolated from aged brain to activate the plasma membrane Ca-ATPase. Biochem Cell Biol 30:9536
Garner MH, Spector A (1980) Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci USA 77:1274–1277
Heys K, Friedrich M, Truscott RJW (2007) Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell 6:807–815
Heys KR, Friedrich MG, Truscott RJW (2008) Free and bound water in normal and cataractous human lenses. Investig Ophthalmol Vis Sci 49:1991–1997
Hill EG, Schwacke JH, Comte-Walters S, Slate EH, Oberg AL, Eckel-Passow JE, Therneau TM, Schey KL (2008) A statistical model for iTRAQ data analysis. J Proteome Res 7:3091–3101
Horwitz J (1992) Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA 89:10449–10453
Horwitz J (2003) Alpha-crystallin. Exp Eye Res 76:145–153
Ji S-R, Wu Y, Sui S-f (2001) Cholesterol is an important factor affecting the membrane insertion of beta-amyloid peptide (A-beta 1-40) which may potentially inhibit the fibril formation. J Biol Chem 277:6273–6279
Koretz JF, Cook CA, Kuszak JR (1994) The zones of discontinuity in the human lens: development and distribution with age. Vis Res 34:2955–2962
Korlimbinis A, Berry Y, Thibault D, Schey KL, Truscott RJW (2008) Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res 88:966–973
Kuszak JR (1995) The development of lens sutures. Prog Retin Eye Res 14:567–591
Lampi KJ, Ma Z, Shih M, Shearer TR, Smith JB, Smith DL, David LL (1997) Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem 272:2268–2275
Lou MF (2000) Thiol regulation in the lens. J Ocul Pharmacol Ther 16:137–148
Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J (2008) Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE 3:e1529
McFall-Ngai MJ, Ding L-L, Takemoto LJ, Horwitz J (1985) Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp Eye Res 41:745–758
McLaurin J, Chakrabartty A (1996) Membrane disruption by alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem 271:26482–26489
Merdes A, Gounari F, Georgatos SD (1993) The 47-kD lens-specific protein phakinin is a tailless intermediate filament protein and an assembly partner of filensin. J Cell Biol 123:1507–1516
Michaelis BDJ, Schöneich C, Williams TD, Ramonda L, Yin D, Hühmer AFR, Yao Y, Gao J, Squier C (1996) Decreased plasma membrane calcium transport activity in aging brain. Life Sci 59:405–412
Michels A, Joisher JN, Hagen TM (2003) Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch Biochem Biophys 410:112–120
Moffat BA, Landman KA, Truscott RJ, Sweeney MH, Pope JM (1999) Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res 69:663–669
Mosevitsky MI (2005) Nerve ending “Signal” proteins GAP-43, MARCKS, and BASP1. In: Kwang WJ (ed) A survey of cell biology. Academic, pp 245–325
Mosevitsky MI, Capony JP, Skladchikova GY, Novitskaya VA, Plekhanov AY, Zakharov VV (1997) The BASP1 family of myristoylated proteins abundant in axonal termini. Primary structure analysis and physico-chemical properties. Biochimie 79:373–384
Mosevitskya MI, Caponyb JP, Skladchikovaa GY, Novitskayaa VA, Yu PA, Zakharov VV (1997) The BASP1 family of myristoylated proteins abundant in axonal termini. Primary structure analysis and physico-chemical properties. Biochimie 79:373–384
Nowakowski RS (2006) Stable neuron numbers from cradle to grave. Proc Natl Acad Sci 103:12219–12220
Prescott AR, Sandilands A, Hutcheson AM, Carter JM, Quinlan RA (1996) The intermediate filament cytoskeleton of the lens: an ever changing network through development and differentiation. A minireview. Ophthalmic Res 28(Suppl 1):58–61
Roy D, Spector A (1976) Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proc Natl Acad Sci USA 73:3484–3487
Schwacke J, Hill E, Krug E, Comte-Walters S, Schey K (2009) iQuantitator: a tool for protein expression inference using iTRAQ. BMC Bioinform 10:342
Shestopalov VI, Bassnett S (2003) Development of a macromolecular diffusion pathway in the lens. J Cell Sci 116:4191–4199
Spalding KL, Bhardwaj RD, Buchholz BA, Druid H (2005) Retrospective birth dating of cells in humans. Cell (Cambridge, Mass) 122:133–143
Spalding KL, Arner E, Westermark PlO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisen J, Arner P (2008) Dynamics of fat cell turnover in humans. Nature (London) 453:783–787
Sweeney MH, Truscott RJ (1998) An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res 67:587–595
Thomson JA, Augusteyn RC (1985) Ontogeny of human lens crystallins. Exp Eye Res 40:393–410
Truscott RJW (2005) Age-related nuclear cataract—oxidation is the key. Exp Eye Res 80:709–725
Wong PT, Schauerte JA, Wisser KC, Ding H, Lee EL, Steel DG, Ari G (2009) Amyloid-beta membrane binding and permeabilization are distinct processes influenced separately by membrane charge and fluidity. J Mol Biol 386:81–96
Zakharov VV, Capony J-P, Derancourt J, Kropolova ES, Novitskaya VA, Bogdanova MN, Mosevitsky MI (2003) Natural N-terminal fragments of brain abundant myristoylated protein BASP1. Biochim Biophys Acta 1622:14–19
Zhang Z, David LL, Smith DL, Smith JB (2001) Resistance of human beta B2-crystallin to in vivo modification. Exp Eye Res 73:203–211
Zieske LR (2006) A perspective on the use of iTRAQ™ reagent technology for protein complex and profiling studies. J Exp Bot 57:1501–1508
Acknowledgements
The authors acknowledge financial support from the NIH EY-013570 (RJWT) and NIH R24 EY14793 as well as support from the MUSC Mass Spectrometry Facility Mr Raj Devasahayam and Meidong Zhu from Sydney Lions Eye bank are thanked for providing the human lenses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
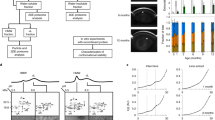
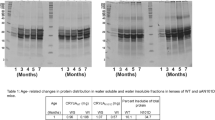
A heat map showing relative changes in major proteins that were tightly bound to lens membranes. Four geometric regions were dissected from each of two lenses aged 20 and 52. The average abundance in the young lens for each region was compared with the same region of the older lens. Red represents an overall relative protein decrease, blue an overall increase and white no change between the two age groups. Proteins with a spectral count less than 15 were not included in the data set. (GIF 94 kb)
About this article
Cite this article
Truscott, R.J.W., Comte-Walters, S., Ablonczy, Z. et al. Tight binding of proteins to membranes from older human cells. AGE 33, 543–554 (2011). https://doi.org/10.1007/s11357-010-9198-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-010-9198-9