Abstract
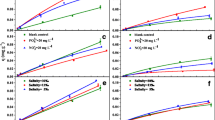
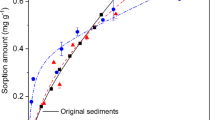
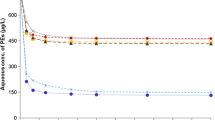
In this study, equilibrium isotherms, kinetics and thermodynamics of ciprofloxacin on seven sediments in a batch sorption process were examined. The effects of contact time, initial ciprofloxacin concentration, temperature and ionic strength on the sorption process were studied. The K d parameter from linear sorption model was determined by linear regression analysis, while the Freundlich and Dubinin–Radushkevich (D–R) sorption models were applied to describe the equilibrium isotherms by linear and nonlinear methods. The estimated K d values varied from 171 to 37,347 mL/g. The obtained values of E (free energy estimated from D-R isotherm model) were between 3.51 and 8.64 kJ/mol, which indicated a physical nature of ciprofloxacin sorption on studied sediments. According to obtained n values as measure of intensity of sorption estimate from Freundlich isotherm model (from 0.69 to 1.442), ciprofloxacin sorption on sediments can be categorized from poor to moderately difficult sorption characteristics. Kinetics data were best fitted by the pseudo-second-order model (R 2 > 0.999). Thermodynamic parameters including the Gibbs free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) were calculated to estimate the nature of ciprofloxacin sorption. Results suggested that sorption on sediments was a spontaneous exothermic process.





Similar content being viewed by others
References
Ahmaruzzaman M, Gayatri SL (2010) Batch adsorption of 4-nitrophenol by acid activated jute stick char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 158:173–180
Belden JB, Maul JD, Lydy MJ (2007) Partitioning and photodegradation of ciprofloxacin in aqueous systems in the presence of organic matter. Chemosphere 66:1390–1395
Białk-Bielińska A, Maszkowska J, Mrozik W, Bielawska A, Kołodziejska M, Palavinskas R, Stepnowski P, Kumirska J (2012) Sulfadimethoxine and sulfaguanidine: their sorption potential on natural soils. Chemosphere 86:1059–1065
Boxall ABA, Kay P, Blackwell PA, Fogg LA (2004) Fate of veterinary medicines applied to soils. In: Kümmerer K (ed) Pharmaceuticals in the environment: sources, fate, effects and risks. Springer-Verlag, Berlin, pp 165–180
Cao X, Pang H, Yang G (2015) Sorption behaviour of norfloxacin on marine sediments. J Soils Sediments 15:1635–1643
Chao Y, Zhu W, Wu X, Hou F, Xun S, Wu P, Ji H, Xu H, Li H (2014) Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem Eng J 243:60–67
Chen H, Ma LQ, Gao B, Gu C (2013) Effects of Cu and Ca cations and Fe/Al coating on ciprofloxacin sorption onto sand media. J Hazard Mater 252–253:375–381
Chowdhury S, Das Saha P (2012) Biosorption of methylene blue from aqueous solutions by a waste biomaterial: hen feathers. Appl Water Sci 2:209–219
Conkle JL, Lattao C, White JR, Cook RL (2010) Competitive sorption and desorption behavior for three fluoroquinolone antibiotics in a wastewater treatment wetland soil. Chemosphere 80:1353–1359
Cordova-Kreylos AL, Scow KM (2007) Effects of ciprofloxacin on salt marsh sediment microbial communities. ISME J 1:585–595
Cueva-Mestanza R, Sosa-Ferrera Z, Torres-Padrón ME, Santana-Rodríguez JJ (2008) Preconcentration of pharmaceuticals residues in sediment samples using microwave assisted micellar extraction coupled with solid phase extraction and their determination by HPLC–UV. J Chromatogr B 863:150–157
de Bruijn B, de Boer B, Van der Mejden AM (1989) Simulation model for the long-term development of water and sediment quality in major Dutch rivers and lakes. Water Pollution Research Report No. 19, CEC. Proceedings of the COST 641 (Working Party 2) Workshop held in Bilthaven, The Netherlands, 20–21 April, 1989
Díaz-Cruz MS, López de Alda MJ, Barceló D (2003) Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. Trac Trend Anal Chem 22:340–351
Domínguez JR, González T, Palo P, Cuerda-Correa EM (2011) Removal of common pharmaceuticals present in surface waters by Amberlite XAD-7 acrylic-ester-resin: influence of pH and presence of other drugs. Desalination 269:231–238
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Chemisches Zentralblatt 1:875–890
EPIweb 4.0 (2016) http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm
Estevez E, Hernandez-Moreno J, Fernandez-Vera JR, Palacios-Diaz MP (2014) Ibuprofen adsorption in four agricultural volcanic soils. Sci Total Environ 468–469:406–414
Fakhri A, Adami S (2014) Adsorption and thermodynamic study of cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J Taiwan Inst Chem Eng 45:1001–1006
Fierro V, Torne-Fernandez V, Montane D, Celzard A (2008) Adsorption of phenol onto activated carbons having different textural and surface properties. Micropor Mesopor Mat 111:276–284
Figueroa-Diva RA, Vasudevan D, MacKay AA (2010) Trends in soil sorption coefficients within common antimicrobial families. Chemosphere 79:786–793
Foo KY, Hameed BH (2010) Insights into the modelling of adsorption isotherm systems. Chem Eng J 156:2–10
Freundlich HMF (1906) Über die Adsorption in Lösungen. Z Phys Chem 57A:385–470
Goldberg S (2005) Equations and models describing adsorption processes in soils, Soil Science Society of America, 677 S. Segoe Road, Madison, WI 53711, USA. Chemical Processes in Soils. SSSA Book Series, no. 8
Guler UA, Sarioglu M (2014) Removal of tetracycline from wastewater using pumice stone: equilibrium, kinetic and thermodynamic studies. J Environ Health Sci Eng 79:1–12
Gupta GS, Prasad G, Panday KK, Singh VN (1988) Removal of chrome dye from aqueous solutions by fly ash. Water Air Soil Pollut 37:13–24
Hadi M, Samarghandi MR, McKay G (2010) Equilibrium two-parameter isotherms of acid dyes sorption by activated carbons: study of residual errors. Chem Eng J 160:408–416
Ho YS, McKay G (1999) The sorption of lead(II) ions on peat. Water Res 33:578–584
Hörsing M, Ledin A, Grabic R, Fick J, Tysklind M, Cour Jansen J, Andersen HR (2011) Determination of sorption of seventy-five pharmaceuticals in sewage sludge. Water Res 45:4470–4482
Huang MH, Yang YD, Chen DH, Chen L, Guo HD (2012) Removal mechanism of trace oxytetracycline by aerobic sludge. Process Saf Environment 90:141–146
Jin L, He M, Zhang J, Xia X (2011) Norfloxacin sorption to different fractions in sediments from typical water systems in China. Soil Sediment Contam 20:564–580
Khattri SD, Singh MK (2000) Colour removal from synthentic dye wastewater using a bioadsorbent. Water Air Soil Pollut 120:283–294
Kim YK, Lim SJ, Han MH, Cho JY (2012) Sorption characteristics of oxytetracycline, amoxicillin, and sulfathiazole in two different soil types. Geoderma 185–186:97–101
Kümmerer K (2009) Antibiotics in the aquatic environment—a review– part I. Chemosphere 75:417–434
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad Handlingar Band 24: 1–39
Lei X, Lu J, Liu Z, Tong Y, Li S (2015) Concentration and distribution of antibiotics in water-sediment system of Bosten Lake, Xinjiang, Environ Sci Pollution Res 22: 1670–1678
Lertpaitoonpan W, Ong SK, Moorman TB (2009) Effect of organic carbon and pH on soil sorption of sulfamethazine. Chemosphere 76:558–564
Li H, Zhang D, Han X, Xing B (2014) Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 95:150–155
Liao X, Zhang C, Yao L, Li J, Liu M, Xu L, Evalde M (2014) Sorption behavior of nonylphenol (NP) on sewage-irrigated soil: kinetic and thermodynamic studies. Sci Total Environ 473–474:530–536
Liu WX, Li WB, Xing BS, Chen JL, Tao S (2011) Sorption isotherms of brominated diphenyl ethers on natural soils with different organic carbon fractions. Environ Pollut 159:2355–2358
Luo Y, Mao D, Rysz M, Zhou Q, Zhang H, Xu L, Alvarez JJP (2010) Trends in antibiotic resistance genes occurrence in the Hai River, China. Environ. Sci. Technol. 44:7220–7225
Maheshwari M, Vyas RK, Sharma M (2013) Kinetics, equilibrium and thermodynamics of ciprofloxacin hydrochloride removal by adsorption on coal fly ash and activated alumina. Desalin Water Treat 51:7241–7254
McKinney CW, Loftin KA, Meyer MT, Davis JG, Pruden A (2010) Tet and sul antibiotic resistance genes in livestock lagoons of various operation type, configuration, and antibiotic occurrence. Environ Sci Technol 44:6102–6109
Mutavdžić Pavlović D, Ćurković L, Blažek D, Župan J (2014) The sorption of sulfamethazine on soil samples: isotherms and error analysis. Sci Total Environ 497–498:543–552
Naslund J, Hedman JE, Agestrand C (2008) Effects of the antibiotic ciprofloxacin on the bacterial community structure and degradation of pyrene in marine sediment. Aquat Toxicol 90:223–227
Ncibi MC (2008) Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J Hazard Mater 153:207–212
Northcott GL, Jones KC (2000) Experimental approaches and analytical techniques for determining organic compound bound residues in soil and sediment. Environ Pollut 108:19–43
OECD (2000) Adsorption-desorption using a batch equilibrium method. OECD Guideline for the Testing of Chemicals 106. Organization for economic Cooperation and Development, Paris, France
Ötker HM, Balcıoğlu IA (2005) Adsorption and degradation of enrofloxacin, a veterinary antibiotic on natural zeolite. J Hazard Mater 122:251–258
Özacar M, Sengýl IA (2004) Two-stage batch sorber design using second-order kinetic model for the sorption of metal complex dyes onto pine sawdust. Biochem Eng J 21:39–45
Pan B, Huang P, Wu M, Wang Z, Wang P, Jiao X, Xing B (2012) Physicochemical and sorption properties of thermally-treated sediments with high organic matter content. Bioresource Technol 103:367–373
Panday KK, Prasad G, Singh VN (1983) Adsorption technique for removal of oxalic-acid. Nat Acad Sci Lett 6:415–417
Patel KS, Patel JC, Dholariya HR, Patel VK, Pate KD (2012) Synthesis of Cu(II), Ni(II), Co(II), and Mn(II) complexes with ciprofloxacin and their evaluation of antimicrobial, antioxidant and anti-tubercular activity. Open J Metal 2:49–59
Pei Z, Shan XQ, Kong J, Wen B, Owens G (2009) Coadsorption of ciprofloxacin and Cu(II) on montmorillonite and kaolinite as affected by solution pH. Environ. Sci. Technol. 44:915–920
Peng Teoh Y, Khan MA, Choong TSY (2013) Kinetic and isotherm studies for lead adsorption from aqueous phase on carbon coated monolith. Chem Eng J 217:248–255
Pereira Leal RM, Ferracciú Alleoni LR, Tornisielo VL, Borges Regitano J (2013) Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 92:979–985
Pereira Leal RM, Figueira RF, Tornisielo VL, Borges Regitano J (2012) Occurrence and sorption of fluoroquinolones in poultry litters and soils from São Paulo State, Brazil, Sci. Total Environ. 432: 344–349
Peruchi LM, Fostier AH, Rath S (2015) Sorption of norfloxacin in soils: analytical method, kinetics and Freundlich isotherms. Chemosphere 119:310–317
Picó Y, Andreu V (2007) Fluoroquinolones in soil—risks and challenges. Anal Bioanal Chem 387:1287–1299
Ramachandran P, Vairamuthu R, Ponnusamy S (2011) Adsorption isotherms, kinetics, thermodynamics and desorption studies of reactive Orange 16 on activated carbon derived from Ananas comosus (L.) carbon. ARPN J Eng Appl Sci 6:15–26
Randhawa NS, Das NN, Jana RK (2014) Adsorptive remediation of Cu(II) and Cd(II) contaminated water using manganese nodule leaching residue. Desalin Water Treat 52:4197–4211
Ross DL, Riley CM (1992) Physicochemical properties of the fluoroquinolone antimicrobials. III. Complexation of lomefloxacin with various metal ions and the effect of metal ion complexation on aqueous solubility. Int J Pharm 87:203–213
Sassman SA, Lee LS (2005) Sorption of three tetracyclines by several soils: assessing the role of pH and cation exchange. Environ Sci Technol 39:7452–7459
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, second edn. John Wiley & Sons, Inc., Hoboken
Schaffer M, Boxberger N, Börnick H, Licha T, Worch E (2012) Sorption influenced transport of ionizable pharmaceuticals onto a natural sandy aquifer sediment at different pH. Chemosphere 87:513–520
Sithole BB, Guy RD (1987) Models for tetracycline in aquatic environments. Water Air Soil Pollut 32:303–314
Srinivasan P, Sarmah AK, Manley-Harris M (2014) Sorption of selected veterinary antibiotics onto dairy farming soils of contrasting nature. Sci Total Environ 472:695–703
Sukul P, Lamshöft M, Zühlke S, Spiteller M (2008) Sorption and desorption of sulfadiazine in soil and soil–manure systems. Chemosphere 73:1344–1350
Thiele-Bruhn S, Seibicke T, Schulten HR, Leinweber P (2004) Sorption of sulfonamide pharmaceutical antibiotics on whole soils and particle-size fractions. J Environ Qual 33:1331–1342
Thiele-Bruhn S (2003) Pharmaceutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sc 166:145–167
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35:3397–3406
Tor A, Cengeloglu Y (2006) Removal of Congo red from aqueous solution by adsorption onto acid activated red mud. J Hazard Mater B138:409–415
Treybal RE (1981) Mass-transfer operations, 3rd ed., McGraw-Hill
Tunali S, Ozcan AS, Ozcan A, Gedikbey T (2006) Kinetics and equilibrium studies for the adsorption of Acid Red 57 from aqueous solutions onto calcined-alunite. J Hazard Mater 135:141–148
Turel I, Bukovec N, Farkas E (1996) Complex formation between some metals and a quinolone family member (ciprofloxacin). Polyhedron 15:269–275
Turiel E, Martín-Esteban A, Tadeo JL (2006) Multiresidue analysis of quinolones and fluoroquinolones in soil by ultrasonic-assisted extraction in small columns and HPLC-UV. Anal Chim Acta 562:30–35
Vasiliu S, Bunia I, Racovita S, Neagu V (2011) Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: kinetics, equilibrium and thermodynamic studies. Carbohyd Polym 85:376–387
Vasudevan D, Bruland GL, Torrance BS, Upchurch VG, Mackay AA (2009) pH-dependent ciprofloxacin sorption to soils: interaction mechanisms and soil factors influencing sorption. Geoderma 151:68–76
Vazquez-Roig P, Segarra R, Blasco C, Andreu V, Picó Y (2010) Determination of pharmaceuticals in soils and sediments by pressurized liquid extraction and liquid chromatography tandem mass spectrometry. J Chromatogr A 1217:2471–2483
Xu WH, Zhang G, Wai OWH, Zou SC, Li XD (2009) Transport and sorption of antibiotics by marine sediments in a dynamic environment. J Soils Sediments 9:364–373
Yang B, Liu S (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159:1877–1885
Yang JF, Ying GG, Zhao JL, Tao R, Su HC, Chen F (2010) Simultaneous determination of four classes of antibiotics in sediments of the Pearl River using RRLC-MS/MS. Sci Total Environ 408:3424–3432
Zhang CL, Qiao GL, Zhao F, Wang Y (2011a) Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J Mol Liq 163:53–56
Zhang L, Song XY, Liu XY, Yang LJ, Pan F, Lv JN (2011b) Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem Eng J 178:26–33
Zhang J, Li ZJ, Ge GF, Sun WC, Liang YC, Wu LS (2009) Impacts of soil organic matter, pH and exogenous copper on sorption behavior of norfloxacin in three soils. J Environ Sci-China 21:632–640
Zhou LJ, Ying GG, Zhao JL, Yang JF, Wang L, Yang B, Liu S (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159:1877–1885
Zrnčić M, Babić S, Mutavdžić Pavlović D (2015) Determination of thermodynamic pKa values of pharmaceuticals from five different groups using capillary electrophoresis. J Sep Sci 38(7):1232–1239
Acknowledgements
This study has been fully supported by the Croatian ScienceFoundation under the project Fate of pharmaceuticals in the environment and during advanced wastewater treatment (PharmaFate) (IP-09-2014-2353).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
The original publication of this paper contains an error. Table 7 headers should have a negative sign: - ΔG°, kJ/mol, − ΔH°, kJ/mol, − ΔS°, kJ/mol. The original article was corrected.
An erratum to this article is available at http://dx.doi.org/10.1007/s11356-017-8834-7.
Rights and permissions
About this article
Cite this article
Mutavdžić Pavlović, D., Ćurković, L., Grčić, I. et al. Isotherm, kinetic, and thermodynamic study of ciprofloxacin sorption on sediments. Environ Sci Pollut Res 24, 10091–10106 (2017). https://doi.org/10.1007/s11356-017-8461-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8461-3