Abstract
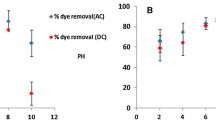
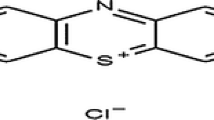
The main purpose of this work is to study the effect of a new process of accelerating which consist to couple the electrochemical process with the adsorption to remove an anionic dye, the indigo carmine. That is why, we investigated the effects of the new process of accelerating the adsorption process by using alternating current (AC) on the retention of an anionic dye, the indigo carmine. The adsorption capacity of dye (mg/g) was raised with the raise of current voltage in solution, temperature, and initial indigo carmine concentration and decreased with the increase of initial solution pH, current density, and mass of carbon. The results demonstrate that the removal efficiency of 97.0 % with the current voltage of 15 V is achieved at a current density of 0.014 A/cm2, of pH 2 using zinc as electrodes and contact time of 210 min for adsorption in the presence of AC. Concerning the adsorption without AC, the results obtained showed that for an initial concentration equal to 20 mg/L, more than 95 % amount of adsorbed dye was retained after 405 min of contact in batch system. The comparison between adsorption in the presence and absence of an alternating current shows the importance of the alternating current in the acceleration of the adsorption method and improve the performances of FILTRASORB 200. For both cases, the adsorption mechanism follows the fractal kinetics BSf(n,α) model and the Brouers–Sotolongo isotherm model provides a good fit of the experimental data for both adsorption with and without alternating current.







Similar content being viewed by others
References
Abidin FCZA, Rahmat NR (2010) Multi-stage ozonation and biological treatment for removal of azo dye industrial effluent. Int J Environ Sci Dev 1(2):193–198
Acosta R, Fierro V, Martinez de Yuso A, Nabarlatz D, Celzard A (2016) Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 149:168–176. doi:10.1016/j.chemosphere.2016.01.093
Ahmad MA, Ahmad N, Bello OS (2015) Adsorption kinetic studies for the removal of synthetic dye using durian seed activated carbon. J Dispers Sci Technol 36:670–684. doi:10.1080/01932691.2014.913983
Alberghina G, Bianchini R, Fichera M, Fisichella S (2000) Dimerization of Cibacron Blue F3GA and other dyes: influence of salts and temperature. Dyes Pigments 46:129–137 jest.2012.42.53
Aliabadi M, Morshedzadeh K, Soheyli H (2006) Removal of hexavalent chromium from aqueous solution by lignocellulosic solid wastes. Int J Environ Sci Technol 3:321–325. doi:10.1007/BF03325940
Aljeboree AM, Alshirifi AN, Alkaim AF (2014) Kinetics and equilibrium study for the adsorptionof textile dyes on coconut shell activated carbon. Arab J Chem. doi:10.1016/j.arabjc.2014.01.020
Al-Khatib L, Fraige F, Al-Hwaiti M, Al-Khashman O (2012) Adsorption from aqueous solution onto natural and acid activated bentonite. Am J Environ Sci 8:510–522. doi:10.3844/ajessp.2012.510.522
Babel S, Opiso ME (2007) Removal of Cr from synthetic wastewater by sorption into volcanic ash soil. Int J Environ Sci Technol 4:99–107. doi:10.1007/BF03325967
Bello OS, Bello IA, Adegoke KA (2013) Adsorption of dyes using different types of sand. A Rev S Afr J Chem 66:117–129
Ben Amor H, Mabrouk A, Talmoudi N (2015) Preparation of activated carbon from date stones:optimization on removal of indigo carmine from aqueous solution using a two-level full factorial design. Int J Eng Res Gen Sci 3:6–17
Ben Douissa N, Dridi-Dhaouadi S, Mhenni MF (2014) Study of antagonistic effect in the simultaneous removal of two textile dyes onto cellulose extracted from Posidonia oceanica using derivative spectrophotometric method. J Water Process Eng 2:1–9. doi:10.1016/j.jwpe.2014.03.004
Ben Hamissa AM, Brouers F, Ncibi MC, Seffen M (2013) Kinetic modeling study on methylene blue sorption onto Agave americana fibers: fractal kinetics and regeneration studies. Sep Sci Technol 48:1–9. doi:10.1080/01496395.2013.809104
Ben Hamissa AM, Lodi A, Seffen M, Finocchio E, Botter R, Converti A (2010) Sorption of Cd(II) and Pb(II) from aqueous solutions onto Agave americana fibers. Chem Eng J 159:67–74. doi:10.1016/j.cej.2010.02.036
Bilgin Simsek E, AvcıTuna AO, Beker U (2015) A statistical approach for arsenic adsorption onto Turkey clinoptilolite. Environ Sci Pollut Res 22:3249–3256. doi:10.1007/s11356-014-2975-8
Bouhamed F, Elouear Z, Bouzid J (2012) Adsorptive removal of copper(II) from aqueous solutions on activated carbon prepared from Tunisian date stones: equilibrium, kinetics and thermodynamics. J Taiwan Inst Chem Eng 43:741–749. doi:10.1016/j.jtice.2012.02.011
Bouhamed F, Elouear Z, Bouzid J, Ouddane B (2015) Multi-component adsorption of copper, nickel and zinc from aqueous solutions onto activated carbon prepared from date stones. Environ Sci Pollut Res (ICIME 2014). doi:10.1007/s11356-015-4400-3
Brouers F (2014a) Statistical foundation of empirical isotherms. Open J Stat 4:687–701. doi:10.4236/ojs.2014.49064
Brouers F (2014b) The fractal (BSf) kinetics equation and its approximations. J Mod Phys 5:1594–1601. doi:10.4236/jmp.2014.516160
Brouers F, Al-Musawi TJ (2015) On the optimal use of of isotherm models for the characterization of biosorption of lead onto algae. J Mol Liq 212:46–51. doi:10.1016/j.molliq.2015.08.054
Brouers F, Sotolongo O, Marquez F, Pirard JP (2005) Microporous and heterogeneous surface adsorption isotherms arising from Levy distributions. Physica A: Stat Mechan Appl 349(1):271–282. doi:10.1016/j.physa.2004.10.032
Brouers F, Sotolongo-Costa O (2006) Generalized fractal kinetics in complex systems (application to biophysics and biotechnology). Physica A 368:165–175. doi:10.1016/j.physa.2005.12.062
Căilean D, Barjoveanu G, Musteret CP, Sulitanu N, Manea LR, Teodosiu C (2009) Reactive dyes removal from wastewater by combined advanced treatment. Environ Eng Manag J, 8(3):503–511. http://omicron.ch.tuiasi.ro/EEMJ/pdfs/vol8/no3/25.
Deo Mall I, Taneja N, Thakur CK (2013) Treatment of indigo carmine dye bearing wastewater by electrocoagulation. 2nd International Conference on Environment, Agriculture and Food Sciences (ICEAFS’2013) August 25–26, 2013 Kuala Lumpur (Malaysia)
El-Ashtoukhy ESZ (2013) Removal of indigo carmine dye from synthetic wastewater by electrochemical oxidation in a new cell with horizontally oriented electrodes. Int J Electrochem Sci 8:846–858
Elkassimi M, Meziane D, Abouarnadasse S, Azizi H (1998) Elimination des colorants de l’industrie de textile par le charbon de bois. Proceeding de la 2ème coférence Maghrébine de Génie des Procédés:555–558
Forgacs E, Cserhatia T, Oros G (2004) Removal of synthetic dyes from wastewaters, a review. Environ Int 30:953–971. doi:10.1016/j.envint.2004.02.001
Freundlich H (1906) Über die adsorption in losungen. Z Phys Chem 57:385–470
García ER, Medina RL, Lozano MM, Pérez IH, Valero MJ, Maubert FAN (2014) Adsorption of azo-dye orange II from aqueous solutions using a metal-organic framework material: iron- benzenetricarboxylate. Materials 7:8037–8057. doi:10.3390/ma7128037
Ghadah A (2014) Removal of Congo red dye from aqueous solution by date palm leaf base. Am J Appl Sci 11(9):1553–1557. doi:10.3844/ajassp.2014.1553.1557
Hammami S, Oturan MA, Oturan N, Bellakhal N, Dachraoui M (2012) Comparative mineralization of textile dye indigo carmine by photo-Fenton process and anodic oxidation using boron-doped diamond anode. Desalin Water Treat 45:297–304
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59(1):171–177. doi:10.1023/B:SCIE.0000013305.99473.cf
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124. doi:10.1016/S0923-0467(98)00076-1
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. doi:10.1016/S0032-9592(98)00112-5
Karthik V, Saravanan K, Bharathi P, Dharanya V, Meiaraj C (2014) An overview of treatments for the removal of textile dyes. J Chem Pharm Sci 7:301–307
Kesraoui A, Moussa A, Ben Ali G, Seffen M (2015) Biosorption of alpacide blue from aqueous solution, by lignocellulosic biomass: Luffa cylindrica fibers. Environ Sci Pollut Res, (ICIME 2014). doi:10.1007/s11356-015-5262-4
Khodaie M, Ghasemi N, Moradi B, Rahimi M (2013) Removal of methylene blue from wastewater by adsorption onto ZnCl2 activated corn husk carbon equilibrium studies. Journal of Chemistry, ID 383985 (2013) 6. Doi:10.1155/2013/383985
Kirupavasam EK, AllenGnana Raj G (2012) Photocatalytic degradation of amido black-10B using nano photocatalyst. J Chem Pharm Res 4(6):2979–2987
Krika F, Azzouz N, Ncibi MC (2012) Removal of hexavalent chromium from aqueous media using Mediterranean Posidonia oceanica biomass: adsorption studies and salt competition investigation. Int J Environ Res 6(3):719–732
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 24(4):1–39
Lakshmi UR, Srivastava VC, Mall ID, Lataye DH (2009) Rice husk ash as an effective adsorbent: evaluation of adsorptive characteristics for indigo carmine dye. J Environ Manag 90(2):710–720. doi:10.1016/j.jenvman.2008.01.002
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc. 2221. Doi: 10.1021/ja02268a002
Low KS, Lee CK, Tan KK (1995) Biosorption of basic dye by water hyacinth roots. Bioresour Technol 52:79–83. doi:10.1016/0960-8524(95)00007-2
Maghri I, Kenz A, Elkouali M, Tanane O, Talbi M (2012) Textile dyes removal from industrial waste water by Mytilus edulis shells. J Mater Environ Sci 3(1):121–136
Mahmoudi K, Hosni K, Hamdi N, Srasra E (2015) Kinetics and equilibrium studies on removal of methylene blue and methyl orange by adsorption onto activated carbon prepared from date pits—a comparative study. Korean J Chem Eng 32(2):274–283. doi:10.1007/s11814-014-0216-y
Malik PK (2004) Dye removal from wastewater using activated carbon developed from sawdust: adsorption equilibrium and kinetics. J Hazard Mater 113:81. doi:10.1016/j.jhazmat.2004.05.022.
Matheswaran M, Karunanithi T (2007) Adsorption of Chrysoidine R by using fly ash in batch process. J Hazard Mater 145(1–2):154–161. doi:10.1016/j.jhazmat.2006.11.006
McKay G, Ramprasad G, Mowli P (1987) Desorption and regeneration of dye colours from low-cost materials. Water Res 21:375–377. http://hdl.handle.net/1783.1/35920.
Mittal J, Mittal L, Kurup L (2006) Batch and bulk removal of hazardous dye, indigo carmine from wastewater through adsorption. J Hazard Mater 137(1):591–602
Namasivayam C, Kavitha D (2002) Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigments 54:47. doi:10.1016/S0143-7208(02)00025-6
Ncibi MC, Altenorc S, Seffen M, Brouerse F, Gaspard S (2008a) Modelling single compound adsorption onto porous and nonporous sorbents using a deformed Weibull exponential isotherm. Chem Eng J 145:196–202. doi:10.1016/j.cej.2008.04.001
Ncibi MC, Mahjoub B, Seffen M (2006) Studies on the biosorption of textile dyes from aqueous solutions using Posidonia oceanica (L.) leaf sheaths fibres. Adsorpt Sci Technol 24:461–473. doi:10.1016/j.jhazmat.2006.06.029
Ncibi MC, Mahjoub B, Seffen M (2007) Kinetic and equilibrium studies of methylene blue biosorption by Posidonia oceanica (L.) fibres. J Hazard Mater B139:280–285. doi:10.7202/019166ar
Ncibi MC, Mahjoub B, Seffen M (2008b) Étude de la biosorption du chrome (VI) par une biomasse méditerranéenne Posidoniaoceanica (L.) delile. Rev Sci Eau J Water Sci 21(4):441–449. doi:10.7202/019166ar
Newcombe G, Drikas M (1997) Adsorption of NOM activated carbon: electro-static and non-electrostatic effects. Carbon 35:1239–1250. doi:10.1016/S0008-6223(97)00078-X
Odogu AN, Daouda K, Desiré BBP, Nsami NJ, Mbadcam KJ (2016) Removal of indigo carmine dye (ic) by batch adsorption method onto dried cola nut shells and its active carbon from aqueous medium. Int J Eng Sci Res Technol 5(3):874–887. doi:10.5281/zenodo.48382
Ong ST, Lee CK, Zainal Z (2007) Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour Technol 98:2792–2799. doi:10.1016/j.biortech.2006.05.011
Ould Brahim I, Belmedani M, Belgacem A, Hadoun H, Sadaoui Z (2014) Discoloration of azo dye solutions by adsorption on activated carbon prepared from the cryogenic grinding of used tires. Chem Eng Trans 38:121–126. doi:10.3303/CET1438021
Rajurkar NS, Desa A (2015) Removal of crystal violet from aqueous solutions using Chitosan and Saraca indica leaves. J Appl Chem. 4(5):1446–1455. http://www.joac.info/
Ramesh TN, Sreenivasa VP (2015) Removal of indigo carmine dye from aqueous solution using magnesium hydroxide as an adsorbent. J Mater 10. doi:10.1155/2015/753057
Rehman R, Zafar J, Nisar H (2014) Adsorption studies of removal of indigo caramine dye from water by formaldehyde and urea treated cellulosic waste of Citrus reticulata peels. Asian J Chem 26(1):43–47. doi:10.14233/ajchem.2014.15305
Rodrigues CS, Madeira LM, Boaventura RA (2013) Treatment of textile dye wastewaters using ferrous sulphate in a chemical coagulation/flocculation process. Environ Technol 34(5–8):719–729. doi:10.1016/j.jhazmat.2009.08.027
Salleh MAM, Mahmoud DK, Karim WA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solidwastes: a comprehensive review. Desalination 280(1–3):1–13. doi:10.1016/j.desal.2011.07.019
Secula S, Cagnon B, Creţescu I, Diaconu M, Petrescu S (2011) Removal of an acid dye from aqueous solutions by adsorption on a commercial granular activated carbon: equilibrium, kinetic and thermodynamic study. M Chem Chem Eng, Biotechnol, Food Ind 12(4):307–322
Shul’gin LP, Kosyakov AL, Kochetkova RD, Petra VI (1975) Inventor’s Certificate N°529124, Byull.Izobret
Sivaramakrishna L, Sivasankar Reddy M, Jagadeesh M, Wan Zuhairi WY, Taha MR, Varada Reddy A (2014) Evaluation of biomass, Indian Jujuba Seed (IJS) for removal of Congo red. Am J Environ Sci 10:374–382. doi:10.3844/ajessp.2014.374.382
Stergiopoulos D, Dermentzis K, Giannakoudakis P, Sotiropoulos S (2014) Electrochemical decolorization and removal of indigo carmine textile dye from wastewater. Glob NEST J 16(3):499–506
Sumanjit K, Rani S, Mahajan RK (2013) Adsorption kinetics for the removal of hazardous dye Congo red by biowaste materials as adsorbents. Journal of Chemistry. ID 628582 (2013) 12
Sun D, Zhang Z, Wang M, Wu Y (2013) Adsorption of reactive dyes on activated carbon developed from Enteromorpha prolifera. Am J Anal Chem 4:17–26. doi:10.4236/ajac.2013.47A003
Saleh TA (2015) Isotherm, kinetic, and thermodynamic studies on Hg(II) adsorption from aqueous solution by silica-multiwall carbon nanotubes. Environ Sci Pollut Res 22:16721–16731. doi:10.1007/s11356-015-4866-z
Temkin MI (1941) Adsorption equilibrium and kinetics of process on non homogeneous surfaces and in the interaction between adsorbed molecules. J Phys Chem 15:296–233
Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA (2013) Graphite oxide/chitosan composite for reactive dye removal. Chem Eng J 217:256–265. doi:10.1016/j.cej.2012.12.008
Ucun H, Bayhan YK, Kaya Y (2008) Kinetic and thermodynamic studies of the biosorption of Cr(VI) by Pinus sylvestris Linn. J Hazard Mater 153:52. doi:10.1016/S0960-8524(02)00086-X
Vargas AMM, Cazetta AL, Kunita MH, Silva TL, Almeida VC (2011) Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chem Eng J 168:722–730. doi:10.1016/j.cej.2011.01.067
Vijayageetha VA, Pandia Rajan A, Arockiaraj SP, Annamalai V, Janakarajan VN, Saravana Balaji MD, Dheenadhayalan MS (2013) Treatment study of dyeing industry effluents using reverse osmosis technology. Research Journal of Recent Sciences ISSN 2277–2502 (3) (ISC-2013) 58–61. www.isca.in
Wang L, Zhang J, Wang A (2008) Removal of methylene blue from aqueous solution using chitosan-g-poly(acrylic acid L)/montmorillonite superadsorbent nanocomposite. Colloids Surf A Physicochem Eng Asp 322(1–3):47–53. doi:10.1016/j.colsurfa.2008.02.019
Yang B, Liu Y, Li Z, Lei L, Zhou J, Zhang X (2016) Preferential adsorption of pentachlorophenol from chlorophenols-containing wastewater using N-doped ordered mesoporous carbon. Environ Sci Pollut Res (2016) 23:1482–1491. doi:10.1007/s11356-015-5384-8
Yurtsever M, Sengil IA (2009) Biosorption of Pb(II) ions by modified quebracho tannin resin. J Hazard Mater 163:58–64. doi:10.1016/j.jhazmat.2008.06.077
Zoughuir H, Khalef H, Bouras O, Chenouf N, Belkaiss D. (1998) Traitement des eaux résiduaires colorées de l’unité de SOITEX de Boufarik par adsorption sur argiles modifiées. Proceeding de la 3ème conférence Maghrébine de Génie des Procédés, Tome 3 pp 296–299
Acknowledgments
The authors of this study express their sincere thanks to the FP4BATIW project and the Laboratory of Energy and Materials for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kesraoui, A., Selmi, T., Seffen, M. et al. Influence of alternating current on the adsorption of indigo carmine. Environ Sci Pollut Res 24, 9940–9950 (2017). https://doi.org/10.1007/s11356-016-7201-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7201-4