Abstract
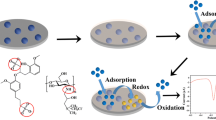
The development and application of a polyaniline/carbon nanotube (CNT) cyclodextrin matrix (PANI-β-CD/MWCNT)-based electrochemical sensor for the quantitative determination of the herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) and its main transformation product 4-chloro-2-methylphenol in natural waters are described. A simple cyclic voltammetry-based electrochemical methodology, in phosphate buffer solution at pH 6.0, was used to develop a method to determine both MCPA and 4-chloro-2-methylphenol, without any previous extraction or derivatization steps. A linear concentration range (10 to 50 μmol L−1) and detection limits of 1.1 and 1.9 μmol L−1, respectively, were achieved using optimized cyclic voltammetric parameters. The proposed method was successfully applied to the determination of MCPA and 4-chloro-2-methylphenol in natural water samples with satisfactory recoveries (94 to 107 %) and in good agreement with the results obtained by an established high-performance liquid chromatography technique, no significant differences being found between the methods. Interferences from ionic species and other herbicides used for broad-leaf weed control were shown to be small. The newly developed methodology was also successfully applied to MCPA photodegradation environmental studies.








Similar content being viewed by others
References
Barceló D, Hennion M-C (1997) Trace determination of pesticides and their degradation products in water. Elsevier, Amsterdam
Belfroid AC, van Drunen M, Beek MA, Schrap SM, van Gestel CAM, van Hattum B (1998) Relative risks of transformation products of pesticides for aquatic ecosystems. Sci Total Environ 222:167–183
Bojanowska-Czajka A, Drzewicz P, Kozyra C, Nałęcz-Jawecki G, Sawicki J, Szostek B, Trojanowicz M (2006) Radiolytic degradation of herbicide 4-chloro-2-methyl phenoxyacetic acid (MCPA) by γ-radiation for environmental protection. Ecotoxicol Environ Saf 65:265–277
Chiron S, Comoretto L, Rinaldi E, Maurino V, Minero C, Vione D (2009) Pesticide by-products in the Rhône delta (Southern France). The case of 4-chloro-2-methylphenol and of its nitroderivative. Chemosphere 74:599–604
Costa C, Maia S, Silva P, Garrido J, Borges F, Garrido EM (2013) Photostabilization of phenoxyacetic acid herbicides MCPA and mecoprop by hydroxypropyl-β-cyclodextrin. Int J Photoenergy 2013, 8 pages
Cserháti T, Forgács E (1998) Phenoxyacetic acids: separation and quantitative determination. J Chromatogr B 717:157–178
Escher BI, Fenner K (2011) Recent advances in environmental risk assessment of transformation products. Environ Sci Technol 45:3835–3847
Eurostat (2007) The use of plant protection products in the European Union: data 1992–2003. Office for official publications of the European Communities, Luxembourg
Gajendran P, Saraswathi R (2008) Polyaniline–carbon nanotube composites. Pure Appl Chem 80:2377–2395
Henriksen T, Svensmark B, Lindhardt B, Juhler RK (2001) Analysis of acidic pesticides using in situ derivatization with alkylchloroformate and solid-phase microextraction (SPME) for GC–MS. Chemosphere 44:1531–1539
Jankowska A, Biesaga M, Drzewicz P, Trojanowicz M, Pyrzynska K (2004) Chromatographic separation of chlorophenoxy acid herbicides and their radiolytic degradation products in water samples. Water Res 38:3259–3264
Laganà A, Bacaloni A, Leva ID, Faberi A, Fago G, Marino A (2002) Occurrence and determination of herbicides and their major transformation products in environmental waters. Anal Chim Acta 462:187–198
Lund H, Baizer MM (1991) Organic electrochemistry, 3rd edn. Marcel Dekker, New York
Mazloum-Ardakani M, Sheikh-Mohseni MA (2011) Carbon nanotubes in electrochemical sensors. In: Naraghi M (ed) Carbon nanotubes—growth and applications. InTech, Rijeka, pp 395–412
Mocak J, Bond AM, Mitchell S, Scollary G (1997) A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: application to voltammetric and stripping techniques (technical report). Pure Appl Chem 69:297–328
Pozo O, Pitarch E, Sancho JV, Hernández F (2001) Determination of the herbicide 4-chloro-2-methylphenoxyacetic acid and its main metabolite, 4-chloro-2-methylphenol in water and soil by liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr A 923:75–85
Rahemi V, Vandamme JJ, Garrido JMPJ, Borges F, Brett CMA, Garrido EMPJ (2012) Enhanced host–guest electrochemical recognition of herbicide MCPA using a β-cyclodextrin carbon nanotube sensor. Talanta 99:288–293
Rahemi V, Garrido JMPJ, Borges F, Brett CMA, Garrido EMPJ (2013) Electrochemical determination of the herbicide bentazone using a carbon nanotube β-cyclodextrin modified electrode. Electroanalysis 25:2360–2366
Regulation (EC) No. 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market. Off J Eur Union L 309/1, 24.11.2009. Version obtained from http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0001:0050:EN:PDF (Last accessed August 2014)
Sapurina IY, Shishov MA (2012) Oxidative polymerization of aniline: molecular synthesis of polyaniline and the formation of supramolecular structures. In: Gomes ADS (ed) New polymers for special applications. InTech, Rijeka, pp 251–312
Shen Q, Wang X (2009) Simultaneous determination of adenine, guanine and thymine based on β-cyclodextrin /MWNTs modified electrode. J Electroanal Chem 632:149–153
Suri CR, Boro R, Nangia Y, Gandhi S, Sharma P, Wangoo N, Rajesh K, Shekhawat GS (2009) Immunoanalytical techniques for analyzing pesticides in the environment. TRAC Trend Anal Chem 28:29–39
Tomassetti M, Martini E, Campanella L (2012) New immunosensors for 2,4-D and 2,4,5-T pesticides determination. Int J Environ Anal Chem 92:417–431
Topalov A, Abramović B, Molnár-Gábor D, Csanádi J, Arcson O (2001) Photocatalytic oxidation of the herbicide (4-chloro-2-methylphenoxy)acetic acid (MCPA) over TiO2. J Photochem Photobiol A Chem 140:249–253
Triegel EK, Guo L (1994) Overview of the fate of pesticides in the environment, water balance; Runoff vs. leaching. In: Mechanisms of pesticide movement into ground water, Ann Arbor. Lewis Publishers, Boca Raton
United Nations Environment Programme (1998), Risk assessment 4-chloro-2-methylphenol, chemicals screening information dataset (SIDS). Version obtained from http://www.inchem.org/documents/sids/sids/1570645.pdf (Last accessed August 2014).
Ureta-Zanãrtu MS, Bustos P, Berríos C, Diez MC, Mora ML, Gutiérrez C (2002) Electrooxidation of 2,4-dichlorophenol and other polychlorinated phenols at a glassy carbon electrode. Electrochim Acta 47:2399–2406
Verma N, Dhillon SS (2003) Biosensors for monitoring insecticides and herbicides—a survey. Int J Environ Stud 60:29–43
Wang Z, Xiao S, Chen Y (2006) β-Cyclodextrin incorporated carbon nanotubes-modified electrodes for simultaneous determination of adenine and guanine. J Electroanal Chem 589:237–242
Yang W, Jiao F, Zhou L, Chen X, Jiang X (2013) Molecularly imprinted polymers coated on multi-walled carbon nanotubes through a simple indirect method for the determination of 2,4-dichlorophenoxyacetic acid in environmental water. Appl Surf Sci 284:692–699
Zertal A, Sehili T, Boule P (2001) Photochemical behaviour of 4-chloro-2-methylphenoxyacetic acid e influence of pH and irradiation wavelength. J Photochem Photobiol A Chem 146:37–48
Acknowledgments
Financial support from Fundação para a Ciência e Tecnologia FCT/MCTES project PTDC/AGR-AAM/105044/2008, National Funds PIDDAC also co-financed by the European Community Fund FEDER through COMPETE–Programa Operacional Factores de Competitividade (POFC), is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Rahemi, V., Garrido, J.M.P.J., Borges, F. et al. Electrochemical sensor for simultaneous determination of herbicide MCPA and its metabolite 4-chloro-2-methylphenol. Application to photodegradation environmental monitoring. Environ Sci Pollut Res 22, 4491–4499 (2015). https://doi.org/10.1007/s11356-014-3693-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3693-y