Abstract
Proteins play essential roles in all aspects of cellular processes, such as biosynthesis, division, growth, motility, metabolism, signaling, and transmission of genetic information. Proteins, however, could deform under mechanical forces, thus altering their biological functions. Here we present protein deformation as a possible molecular basis for mechanosensing and mechanotransduction, elucidate the important features of protein mechanics including protein deformation mode and dynamics, illustrate how protein deformation could alter biological function, and describe the important roles of protein deformation in force-sensing, force transducing and mechanochemical coupling in cells. The experimental and modeling challenges in protein mechanics are discussed.






Similar content being viewed by others
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (2002) Molecular biology of the cell, 4th edn. Garland, New York, NY.
Wang N, Butler JP Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260:1124–1127. doi:10.1126/science.7684161.
Vogel V, Sheetz M (2006) Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7:265–275. doi:10.1038/nrm1890.
Howard J (2001) Mechanics of motor proteins and the cytoskeleton. Sinauer, Sunderland, MA.
Block SM, Goldstein LS, et al (1990) Bead movement by single kinesin molecules studied with optical tweezers. Nature 348:348–352. doi:10.1038/348348a0.
Stossel TP (1993) On the crawling of animal cells. Science 260:1086–1094. doi:10.1126/science.8493552.
Pelham RJ, Wang Y (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94:13661–13665. doi:10.1073/pnas.94.25.13661.
Discher DE, Janmey P, Wang Y (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143. doi:10.1126/science.1116995.
Brownell WE, Spector AA, Raphael RM, Popel AS (2001) Micro- and nanomechanics of the cochlear outer hair cell. Annu Rev Biomed Eng 3:169–194. doi:10.1146/annurev.bioeng.3.1.169.
Bao G (2002) Mechanics of biomolecules. J Mech Phys Solids 50:2237–2274. doi:10.1016/S0022-5096(02)00035-2.
Zhu C, Bao G, Wang N (2000) Cell mechanics: mechanical response, cell adhesion, and molecular deformation. Annu Rev Biomed Eng 2:189–226. doi:10.1146/annurev.bioeng.2.1.189.
Bershadsky AD, Balaban NQ, Geiger B (2003) Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19:677–695. doi:10.1146/annurev.cellbio.19.111301.153011.
Bershadsky A, Kozlov M, Geiger B (2006) Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol 18:472–481. doi:10.1016/j.ceb.2006.08.012.
Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S (2001) Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA 98:1042–1046. doi:10.1073/pnas.031562998.
Fung YC (1990) Biomechanics: motion, flow, stress, and growth. Springer, New York.
Fung YC (1993) Biomechanics: mechanical properties of living tissues, 2nd edn. Springer, New York.
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689. doi:10.1016/j.cell.2006.06.044.
Ali MH, Schumacker PT (2002) Endothelial responses to mechanical stress: where is the mechanosensor? Crit Care Med 30:S198–S206. doi:10.1097/00003246-200205001-00005.
Wootton DM, Ku DN (1999) Fluid mechanics of vascular systems, diseases, and thrombosis. Annu Rev Biomed Eng 1:299–329. doi:10.1146/annurev.bioeng.1.1.299.
Fisher AB, Chien S, Barakat AI, Nerem RM (2001) Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol 281:L529–L533.
Hu S, Sachs F (1997) Stretch-activated ion channels in the heart. J Mol Cell Cardiol 29:1511–1523. doi:10.1006/jmcc.1997.0392.
Ingber DE (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91:877–887. doi:10.1161/01.RES.0000039537.73816.E5.
Creighton TE (1993) Proteins. Freeman, New York, NY.
Bao G, Suresh S (2003) Cell and molecular mechanics of biological materials. Nat Matters 2:715–726. doi:10.1038/nmat1001.
Voet D, Voet JG (1995) Biochemistry, 2nd edn. Wiley, New York, NY.
McCammon JA, Gelin BR, Karplus M, Wolynes PG (1976) The hinge-bending mode in lysozyme. Nature 262:325–326. doi:10.1038/262325a0.
Rief M, Gautel M, Oesterhelt F, Fernandez JM, Gaub H (1997) Reversible unfolding of individual titin immunoglobulin domains by AFM. Science 276:1109–1112. doi:10.1126/science.276.5315.1109.
Kellermayer MSZ, Smith SB, Granzier HL, Bustamante C (1997) Folding–unfolding transitions in single titin molecules characterized with laser tweezers. Science 276:1112–1116. doi:10.1126/science.276.5315.1112.
Tskhovrebova L, Trinnick J, Sleep JA, Simmons RM (1997) Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature 387:308–312. doi:10.1038/387308a0.
Oberhauser AF, Marszalek PE, Erickson HP, Fernandez JM (1998) The molecular elasticity of the extracellular matrix protein tenascin. Nature 393:181–185. doi:10.1038/30270.
Krammer A, Lu H, Isralewitz B, Schulten K, Vogel V (1999) Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc Natl Acad Sci USA 96:1351–1356. doi:10.1073/pnas.96.4.1351.
Ohashi T, Kiehart DP, Ericson H (1999) Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc Natl Acad Sci USA 96:2153–2158.
Craig D, Krammer A, Schulten K, Vogel V (2001) Comparison of the early stages of forced unfolding for fibronectin type III modules. Proc Natl Acad Sci USA 98:5590–5595. doi:10.1073/pnas.101582198.
Vogel V, Thomas WE, Craig DW, Krammer A, Baneyx G (2001) Structural insights into the mechanical regulation of molecular recognition sites. Trends Biotechnol 19:416–423. doi:10.1016/S0167-7799(01)01737-1.
Puklin-Faucher E, Gao M, Schulten K, Vogel V (2006) How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J Cell Biol 175:349–360. doi:10.1083/jcb.200602071.
Ruggeri ZM (2003) von Willebrand factor. Curr Opin Hematol 10:142–149. doi:10.1097/00062752-200303000-00008.
Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE (2007) Forced unfolding of proteins within cells. Science 317:663–666. doi:10.1126/science.1139857.
Soto C (2001) Protein misfolding and disease; protein refolding and therapy. FEBS Lett 498:204–207. doi:10.1016/S0014-5793(01)02486-3.
Marko JF, Siggia ED (1995) Stretching DNA. Macromolecules 28:8759–8770. doi:10.1021/ma00130a008.
Schwaiger I, Schleicher M, Noegel AA, Rief M (2005) The folding pathway of a fast-folding immunoglobulin domain revealed by single-molecule mechanical experiments. EMBO Rep 6:46–51. doi:10.1038/sj.embor.7400317.
Merkel R, Nassoy P, Leung A, Ritchie K, Evans E (1999) Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy. Nature 397:50–53. doi:10.1038/16219.
Subbiah S (1996) Protein motions. Chapman & Hall, Austin, TX.
van Kampen NG (1981) Stochastic processes in physics and chemistry. North-Holland, New York, NY.
Risken H (1984) The fokker-planck equation: methods of solution and applications, 2nd edn. Springer, New York, NY.
Uhlenbeck GE, Ornstein LS (1930) On the theory of the Brownian motion. Phys Rev 36:823–841. doi:10.1103/PhysRev.36.823.
Boyer PD (1993) The binding change mechanism for ATP synthase—some probabilities and possibilities. Biochem Biophys Acta 1140:215–250. doi:10.1016/0005-2728(93)90063-L.
Lauffenburger DA, Linderman JJ (1993) Receptors. Oxford University Press, New York.
Israelachvili J (1992) Intermolecular and surface forces. Academic, San Diego, CA.
Evans E, Ritchie K (1997) Dynamic strength of molecular adhesion bonds. Biophys J 72:1541–1555.
Sanchez-Mateos P, Cabanas C, Sanchez-Madrid F (1996) Regulation of integrin function. Semin Cancer Biol 7:99–109. doi:10.1006/scbi.1996.0015.
Hirokawa N (1998) Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279:519–526. doi:10.1126/science.279.5350.519.
Mermall V, Post PL, Mooseker MS (1998) Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 279:527–533. doi:10.1126/science.279.5350.527.
Cramer P, Bushnell DA, Fu J, Gnatt AL, Maier-Davis B, Thompson NE, Burgess RR, Edwards AM, David PR, Kornberg RD (2000) Architecture of RNA polymerase II and implications for the transcription mechanism. Science 288:640–649. doi:10.1126/science.288.5466.640.
Walker ML, Burgess SA, Sellers JR, Wang F, Hammer JA 3rd, Trinick J, Knight PJ (2000) Two-headed binding of a processive myosin to F-actin. Nature 405:804–807. doi:10.1038/35015592.
Vale RD, Milligan RA (2000) The way things move: looking under the hood of molecular motor proteins. Science 288:88–95. doi:10.1126/science.288.5463.88.
Meyhofer E, Howard J (1995) The force generated by a single kinesin molecule against an elastic load. Proc Natl Acad Sci USA 92:574–578. doi:10.1073/pnas.92.2.574.
Yasuda R, Noji H, Yoshida M, Kinosita K Jr, Itoh H (2001) Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature 410:898–904. doi:10.1038/35073513.
Kinosita K Jr, Yasuda R, Noji H (2000) F1-ATPase: a highly efficient rotary ATP machine. Essays Biochem 35:3–18.
Soong RK, Bachand GD, Neves HP, Olkhovets AG, Craighead HG, Montemegno CD (2000) Powering an inorganic nanodevice with a biomolecular motor. Science 290:1555–1558. doi:10.1126/science.290.5496.1555.
Elston T, Wang H, Oster G (1998) Energy transduction in ATP synthase. Nature 391:510–513. doi:10.1038/35185.
Wang H, Oster G (1998) Energy transduction in the F1 motor of ATP synthase. Nature 396:279–282. doi:10.1038/24409.
Baneyx G, Baugh L, Vogel V (2001) Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc Natl Acad Sci USA 98:14464–14468. doi:10.1073/pnas.251422998.
Baneyx G, Baugh L, Vogel V (2002) Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA 99:5139–5143. doi:10.1073/pnas.072650799.
Acknowledgements
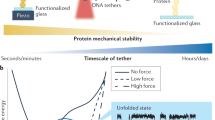
This work was supported by the National Heart Lung and Blood Institute of the NIH as a Program of Excellence in Nanotechnology (HL80711) and the NIH Roadmap Initiative in Nanomedicine through a Nanomedicine Development Center award (EY018244). The author would like to thank Dr. Wonjong Rhee for generating the results shown in Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, G. Protein Mechanics: A New Frontier in Biomechanics. Exp Mech 49, 153–164 (2009). https://doi.org/10.1007/s11340-008-9154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-008-9154-0