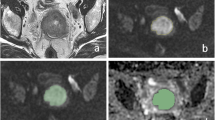
Abstract
Purpose
Apparent diffusion coefficient (ADC) histogram analysis has been used to some extent in cervical cancer (CC) to distinguish between low-grade and high-grade tumors. Although this differentiation is undoubtedly helpful, it would be even more crucial in the presurgical setting to determine whether a tumor already gained the potential to metastasize via the lymphatic system. So far, no studies investigated the potential of 3T ADC histogram analysis in CC to differentiate between nodal-positive and nodal-negative entities. Therefore, the principal aim of our study was to investigate the potential of 3T ADC histogram analysis to differentiate between CC with and without lymph node metastasis. The second aim was to elucidate possible differences in ADC histogram parameters between CC with limited vs. advanced tumor stages and well-differentiated vs. undifferentiated lesions. Finally, correlations of p53 expression and Ki-67 index with ADC parameters were analyzed.
Procedures
Eighteen female patients (mean age 55.4 years, range 32–79 years) with histopathologically confirmed cervical squamous cell carcinoma of the uterine cervix were prospectively enrolled. Tumor stages, tumor grading, status of metastatic dissemination, Ki67-index, and p53 expression were assessed in these patients. Diffusion weighted imaging (DWI) was obtained in a 3T scanner using the following b values: b0 and b1000 s/mm2.
Results
Group comparisons using Mann-Whitney U test revealed the following findings: nodal-positive CC had statistically significant lower ADC parameters (ADCmin, ADCmean, median ADC, Mode, p10, p25, p75, and p90) in comparison to nodal-negative CC (all p < 0.05). ADCentropy was significantly elevated (p = 0.046) in tumors with advanced T stages (T3/4) compared to tumors with limited T stage (T2). ADCmin values were different in a statistically significant manner comparing G1/G2 and G3 tumors (40.45 ± 18.63 vs. 65.0 ± 23.63 × 10–5 mm2 s−1, p = 0.035). Furthermore, Spearman Rho calculation identified an inverse correlation between ADCentropy and p53 expression (r = −0.472, p = 0.048).
Conclusion
The main finding of our study is the discriminability of nodal-positive from nodal-negative CC using ADC histogram analysis in 3T DWI. This information is crucial for the gynecological surgeon to identify the optimal treatment strategy for patients suffering from CC. Furthermore, ADCentropy was identified as a potential imaging biomarker for tumor heterogeneity and might be able to indicate further molecular changes like loss of p53 expression, which is associated with EMT and consequentially indicates a poor prognosis in CC. Finally, our study confirmed the findings of previous works, which indicated that histogram analysis of ADC maps can distinguish between low-grade and high-grade CC. In conclusion, it can be stated that ADC histogram analysis provides additional, prognostically important information on tumor biology in CC.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Waggoner SE (2003) Cervical cancer. Lancet 361:2217–2225
Aoki Y, Sasaki M, Watanabe M et al (2000) High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol 77:305–309
Sakuragi N (2007) Up-to-date management of lymph node metastasis and the role of tailored lymphadenectomy in cervical cancer. Int J Clin Oncol 12:165–175
Zhou Y, Huang Y, Cao X et al (2016) WNT2 promotes cervical carcinoma metastasis and induction of epithelial-mesenchymal transition. PLoS One. doi:10.1371/journal.pone.0160414
Gong Y, Wang Q, Dong L et al (2016) Different imaging techniques for the detection of pelvic lymph nodes metastasis from gynecological malignancies: a systematic review and meta-analysis. Oncotarget. doi:10.18632/oncotarget.12959
Choi HJ, Roh JW, Seo S-S et al (2006) Comparison of the accuracy of magnetic resonance imaging and positron emission tomography/computed tomography in the presurgical detection of lymph node metastases in patients with uterine cervical carcinoma. Cancer 106:914–922
Schob S, Meyer J, Gawlitza M et al (2016a) Diffusion-weighted MRI reflects proliferative activity in primary CNS lymphoma. PLoS One 11:e0161386. doi:10.1371/journal.pone.0161386
Surov A, Ginat DT, Sanverdi E et al (2016) Use of diffusion weighted imaging in differentiating between malignant and benign meningiomas. A multicenter analysis. WNEU 88:598–602
Schob S, Surov A, Wienke A et al (2016b) Correlation between aquaporin 4 expression and different DWI parameters in grade I meningioma. Mol Imaging Biol 19:138–142
Partridge SC, Mullins CD, Kurland BF et al (2010) Apparent diffusion coefficient values for discriminating benign and malignant breast MRI lesions: effects of lesion type and size. AJR Am J Roentgenol 194:1664–1673
Shi HF, Feng Q, Qiang JW et al (2013) Utility of diffusion-weighted imaging in differentiating malignant from benign thyroid nodules with magnetic resonance imaging and pathologic correlation. J Comput Assist Tomogr 37:505–510
Schob S, Voigt P, Bure L et al (2016c) Diffusion-weighted imaging using a readout-segmented, Multishot EPI sequence at 3 T distinguishes between morphologically differentiated and undifferentiated subtypes of thyroid carcinoma—a preliminary study. TRANON 9:403–410
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213
Guan Y, Li W, Jiang Z et al (2016) Whole-lesion apparent diffusion coefficient-based entropy-related parameters for characterizing cervical cancers. Acad Radiol 23:1559–1567
Xue H, Ren C, Yang J et al (2014) Histogram analysis of apparent diffusion coefficient for the assessment of local aggressiveness of cervical cancer. Arch Gynecol Obstet 290:341–348
Heo SH, Shin SS, Kim JW et al (2013a) Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol 14:616–610
Downey K, Riches SF, Morgan VA et al (2013a) Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. AJR Am J Roentgenol 200:314–320
Downey K, Riches SF, Morgan VA et al (2013b) Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. Am J Roentgenol 200:314–320
Heo SH, Shin SS, Kim JW et al (2013b) Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol 14:616–625
Surov A, Caysa H, Wienke A et al (2015) Correlation between different ADC fractions, cell count, Ki-67, total nucleic areas and average nucleic areas in meningothelial meningiomas. Anticancer Res 35:6841–6846
Steinestel K, Eder S, Schrader AJ, Steinestel J (2014) Clinical significance of epithelial-mesenchymal transition. Clin Transl Med. doi:10.1186/2001-1326-3-17
Rojas-Puentes L, Cardona AF, Carranza H et al (2016) Epithelial-mesenchymal transition, proliferation, and angiogenesis in locally advanced cervical cancer treated with chemoradiotherapy. Cancer Med 5:1989–1999
Chen Y-W, Pan H-B, Tseng H-H et al (2013a) Differentiated epithelial- and mesenchymal-like phenotypes in subcutaneous mouse xenografts using diffusion weighted-magnetic resonance imaging. Int J Mol Sci 14:21943–21959
Rosenkrantz AB (2013) Histogram-based apparent diffusion coefficient analysis: an emerging tool for cervical cancer characterization? AJR Am J Roentgenol 200:311–313
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Chang C-J, Chao C-H, Xia W et al (2011) p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 13:317–323
Kim T, Veronese A, Pichiorri F et al (2011) p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 208:875–883
Chen L, Liu M, Bao J et al (2013b) The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS One. doi:10.1371/journal.pone.0079008.s001
Chen L, Zhang J, Chen Y et al (2014) Relationship between apparent diffusion coefficient and tumour cellularity in lung cancer. PLoS One. doi:10.1371/journal.pone.0099865
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Stefan Schob and Hans Jonas Meyer contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Schob, S., Meyer, H.J., Pazaitis, N. et al. ADC Histogram Analysis of Cervical Cancer Aids Detecting Lymphatic Metastases—a Preliminary Study. Mol Imaging Biol 19, 953–962 (2017). https://doi.org/10.1007/s11307-017-1073-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1073-y