Abstract
Purpose
Non-invasive response monitoring can potentially be used to guide therapy selection for breast cancer patients. We employed dynamic 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography ([18F]FDG PET) to evaluate changes in three breast cancer xenograft lines in mice following three chemotherapy regimens.
Procedures
Sixty-six athymic nude mice bearing bilateral breast cancer xenografts (two basal-like and one luminal-like subtype) underwent three 60 min [18F]FDG PET scans. Scans were performed prior to and 3 and 10 days after treatment with doxorubicin, paclitaxel, or carboplatin. Tumor growth was monitored in parallel. A pharmacokinetic compartmental model was fitted to the tumor uptake curves, providing estimates of transfer rates between the vascular, non-metabolized, and metabolized compartments. Early and late standardized uptake values (SUVE and SUVL, respectively); the rate constants k 1, k 2, and k 3, and the intravascular fraction v B were estimated. Changes in tumor volume were used as a response measure. Multivariate partial least-squares regression (PLSR) was used to assess if PET parameters could model tumor response and to identify PET parameters with the largest impact on response.
Results
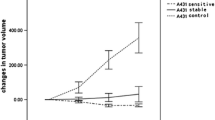
Treatment responders had significantly larger perfusion-related parameters (k 1 and k 2) and lower metabolism-related parameter (k 3) than non-responders 10 days after the start of treatment. These findings were further supported by the PLSR analysis, which showed that k 1 and k 2 at day 10 and changes in k 3 explained most of the variability in response to therapy, whereas SUVL and particularly SUVE were of lesser importance.
Conclusions
Overall, rate parameters related to both tumor perfusion and metabolism were associated with tumor response. Conventional metrics of [18F]FDG uptake such as SUVE and SUVL apparently had little relation to tumor response, thus necessitating full dynamic scanning and pharmacokinetic analysis for optimal evaluation of chemotherapy-induced changes in breast cancers.






Similar content being viewed by others
References
Weigel MT, Dowsett M (2010) Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer 17:R245–R262
Perou CM, Sorlie T, Eisen MB, et al. (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sørlie T, Perou CM, Tibshirani R, et al. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 98:10869–10874
Sørlie T, Tibshirani R, Parker J, et al. (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100:8418–8423
Tannock I (2001) Tumor physiology and drug resistance. Cancer Metastasis Rev 20:123–132
An YY, Kim SH, Kang BJ, Lee AW (2015) Treatment response evaluation of breast cancer after neoadjuvant chemotherapy and usefulness of the imaging parameters of MRI and PET/CT. J Korean Med Sci 30:808–815
Kubota K (2001) From tumor biology to clinical pet: a review of positron emission tomography (PET) in oncology. Ann Nucl Med 15:471–486
El Naqa I, Grigsby P, Apte A, et al. (2009) Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recogn 42:1162–1171
Yoshioka T, Takahashi H, Oikawa H, et al. (1997) Influence of chemotherapy on FDG uptake by human cancer xenografts in nude mice. J Nucl Med 38:714–717
Humbert O, Berriolo-Riedinger A, Cochet A, et al. (2014) Prognostic relevance at 5 years of the early monitoring of neoadjuvant chemotherapy using (18)F-FDG PET in luminal HER2-negative breast cancer. Eur J Nucl Med Mol Imaging 41:416–427
Watabe H, Ikoma Y, Kimura Y, Naganawa M, Shidahara M (2006) PET kinetic analysis—compartmental model. Ann Nucl Med 20:583–588
Dunnwald LK, Doot RK, Specht JM, et al. (2011) PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res 17:2400–2409
Humbert O, Cochet A, Coudert B, et al. (2015) Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist 20:94–104
Kasamon YL, Jones RJ, Wahl RL (2007) Integrating PET and PET/CT into the risk-adapted therapy of lymphoma. J Nucl Med 48(Suppl 1):19s–27s
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122s–150s
Lind P, Igerc I, Beyer T, Reinprecht P, Hausegger K (2004) Advantages and limitations of FDG PET in the follow-up of breast cancer. Eur J Nucl Med Mol Imaging 31(Suppl 1):S125–S134
Lim I, Noh WC, Park J, et al. (2014) The combination of FDG PET and dynamic contrast-enhanced MRI improves the prediction of disease-free survival in patients with advanced breast cancer after the first cycle of neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 41:1852–1860
Semple SI, Staff RT, Heys SD, et al. (2006) Baseline MRI delivery characteristics predict change in invasive ductal breast carcinoma PET metabolism as a result of primary chemotherapy administration. Ann Oncol 17:1393–1398
Malinen E, Rodal J, Knudtsen IS, Sovik A, Skogmo HK (2011) Spatiotemporal analysis of tumor uptake patterns in dynamic (18)FDG-PET and dynamic contrast enhanced CT. Acta Oncol 50:873–882
Mullani NA, Herbst RS, O'Neil RG, Gould KL, Barron BJ, Abbruzzese JL (2008) Tumor blood flow measured by PET dynamic imaging of first-pass 18F-FDG uptake: a comparison with 15O-labeled water-measured blood flow. J Nucl Med 49:517–523
Kristian A, Revheim ME, Qu H, et al. (2013) Dynamic (18)F-FDG-PET for monitoring treatment effect following anti-angiogenic therapy in triple-negative breast cancer xenografts. Acta Oncol 52:1566–1572
Bergamaschi A, Hjortland GO, Triulzi T, et al. (2009) Molecular profiling and characterization of luminal-like and basal-like in vivo breast cancer xenograft models. Mol Oncol 3:469–482
Marangoni E, Vincent-Salomon A, Auger N, et al. (2007) A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 13:3989–3998
Roe K, Aleksandersen TB, Kristian A, et al. (2010) Preclinical dynamic 18F-FDG PET—tumor characterization and radiotherapy response assessment by kinetic compartment analysis. Acta Oncol 49:914–921
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst 58:109–130
Esbensen KH, Guyot D, Westad F, Houmøller LP, Camo ASA (2001) Multivariate Calibration (PCR/PLSR). In Multivariate data analysis—in practice: an introduction to multivariate data analysis and experimental design. Oslo: Camo, pp 115–170
Specht JM, Kurland BF, Montgomery SK, et al. (2010) Tumor metabolism and blood flow as assessed by PET varies by tumor subtype in locally advanced breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 16:2803–2810
Dunnwald LK, Gralow JR, Ellis GK, et al. (2008) Tumor metabolism and blood flow changes by positron emission tomography: relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol 26:4449–4457
Cochet A, Pigeonnat S, Khoury B, et al. (2012) Evaluation of breast tumor blood flow with dynamic first-pass 18F-FDG PET/CT: comparison with angiogenesis markers and prognostic factors. J Nucl Med 53:512–520
Kristian A, Nilsen LB, Roe K, et al. (2013) Dynamic (18) F-FDG PET for assessment of tumor physiology in two breast carcinoma xenografts. Nucl Med Mol Imaging 47:173–180
Acknowledgments
Financial support received from the K.G. Jebsen Center for Breast Cancer Research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kristian, A., Holtedahl, J.E., Torheim, T. et al. Dynamic 2-Deoxy-2-[18F]Fluoro-D-Glucose Positron Emission Tomography for Chemotherapy Response Monitoring of Breast Cancer Xenografts. Mol Imaging Biol 19, 271–279 (2017). https://doi.org/10.1007/s11307-016-0998-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0998-x