Abstract
Purpose
The feasibility of iron oxide nanoparticles (IONPs) conjugated with anti-epidermal growth factor receptor 2 (HER2) single-chain antibody (scFv-IONPs) as novel HER2-targeted magnetic resonance (MR) contrast agents was investigated.
Procedures
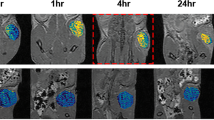
The scFv-IONPs were prepared and identified. For in vitro MRI, NCI-N87 (HER2 high expression) and SUIT2 (low expression) cells were incubated with scFv-IONPs. For in vivo MRI, NCI-N87 and SUIT2 tumor-bearing mice were intravenously injected with scFv-IONPs and imaged before and 24 h post-injection.
Results
The scFv-IONPs demonstrated high transverse relaxivity (296.3 s−1 mM−1) and affinity toward HER2 (KD = 11.7 nM). In the in vitro MRI, NCI-N87 cells treated with scFv-IONPs exhibited significant MR signal reduction (44.6 %) than SUIT2 cells (6.8 %). In the in vivo MRI, decrease of MR signals in NCI-N87 tumors (19.3 %) was more notable than that in SUIT2 tumors (6.2 %).
Conclusions
The scFv-IONPs enabled HER2-specific tumor MR imaging, suggesting the potential of scFv-IONPs as a robust HER2-targeted MR contrast agent.




Similar content being viewed by others
References
Thomas R, Park IK, Jeong YY (2013) Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int J Mol Sci 14:15910–15930
Geraldes CF, Laurent S (2009) Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol Imaging 4:1–23
Wagner V, Dullaart A, Bock AK et al (2006) The emerging nanomedicine landscape. Nat Biotechnol 24:1211–1217
Wang YX, Hussain SM, Krestin GP (2001) Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 11:2319–2331
Wang YX, Xuan S, Port M et al (2013) Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr Pharm Des 19:6575–6593
Ittrich H, Peldschus K, Raabe N et al (2013) Superparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapy. Rofo 185:1149–1166
Wahajuddin AS (2012) Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine 7:3445–3471
McFadden C, Mallett CL, Foster PJ (2011) Labeling of multiple cell lines using a new iron oxide agent for cell tracking by MRI. Contrast Media Mol Imaging 6:514–522
Watada Y, Yamashita D, Toyoda M et al (2015) Magnetic resonance monitoring of superparamagnetic iron oxide (SPIO)-labeled stem cells transplanted into the inner ear. Neurosci Res 95:21–26
Rudin M, Rausch M, Stoeckli M (2005) Molecular imaging in drug discovery and development: potential and limitations of nonnuclear methods. Mol Imaging Biol 7:5–13
Kanazaki K, Sano K, Makino A et al (2015) Development of anti-HER2 fragment antibody conjugated to iron oxide nanoparticles for in vivo HER2-targeted photoacoustic tumor imaging. Nanomedicine 11:2051–2060
Slamon DJ, Godolphin W, Jones LA et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712
Chen TJ, Cheng TH, Chen CY et al (2009) Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem 14:253–260
Rasaneh S, Rajabi H, Babaei MH et al (2011) MRI contrast agent for molecular imaging of the HER2/neu receptor using targeted magnetic nanoparticles. J Nanopart Res 13:2285–2293
Gao J, Chen K, Miao Z et al (2010) Affibody-based nanoprobes for HER2-expressing cell and tumor imaging. Biomaterials 32:2141–2148
Satpathy M, Wang L, Zielinski R et al (2014) Active targeting using HER-2-affibody-conjugated nanoparticles enabled sensitive and specific imaging of orthotopic HER-2 positive ovarian tumors. Small 10:544–555
Elias DR, Cheng Z, Tsourkas A (2010) An intein-mediated site-specific click conjugation strategy for improved tumor targeting of nanoparticle systems. Small 6:2460–2468
Moore A, Weissleder R, Bogdanov A (1997) Uptake of dextran- coated monocrystalline iron oxides in tumor cells and macrophages. J Magn Reson Imaging 7:1140–1145
Keliher EJ, Yoo J, Nahrendorf M et al (2011) 89Zr-labeled dextran nanoparticles enable in vivo macrophage imaging. Bioconjug Chem 22:2383–2389
Perrault SD, Walkey C, Jennings T et al (2009) Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett 9:1909–1915
Chua TC, Merrett ND (2012) Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes—A systematic review. Int J Cancer 130:2845–2856
Gómez-Martin C, Garralda E, Echarri MJ et al (2012) HER2/neu testing for anti-HER2-based therapies in patients with unresectable and/or metastatic gastric cancer. J Clin Pathol 65:751–757
Hedner C, Tran L, Borg D et al (2015) Discordant human epidermal growth factor receptor 2 overexpression in primary and metastatic upper gastrointestinal adenocarcinoma signifies poor prognosis. Histopathology. doi:10.1111/his.12744
Bang YJ, Van Cutsem E, Feyereislova A, Investigators TGAT et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Park JY, Dunbar KB, Vemulapalli R et al (2015) Human epidermal growth factor receptor 2 testing in gastric and gastroesophageal junction adenocarcinomas: role of the gastroenterologist. Gastrointest Endosc 81:977–982
Tanner M, Hollmen M, Junttila TT et al (2005) Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 16:273–278
Yan B, Yau EX, Omar SSB et al (2010) A study of HER2 gene amplification and protein expression in gastric cancer. J Clin Pathol 63:839–842
Ito A, Kuga Y, Honda H et al (2004) Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett 212:167–175
Sonvico F, Mornet S, Vasseur S et al (2005) Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconjug Chem 16:1181–1188
Sadhukha T, Wiedmann TS, Panyam J (2013) Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 34:5163–5171
Acknowledgments
This work was partly supported by the Innovative Techno-Hub for Integrated Medical Bio-imaging Project of the Special Coordination Funds for Promoting Science and Technology, from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Ning Ding and Kohei Sano contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental 1
(PDF 335 kb)
Rights and permissions
About this article
Cite this article
Ding, N., Sano, K., Kanazaki, K. et al. In Vivo HER2-Targeted Magnetic Resonance Tumor Imaging Using Iron Oxide Nanoparticles Conjugated with Anti-HER2 Fragment Antibody. Mol Imaging Biol 18, 870–876 (2016). https://doi.org/10.1007/s11307-016-0977-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0977-2