Abstract
Purpose
Mesenchymal stromal cells (MSCs) hold promise in the treatment of liver disease. However, short survival time of MSCs after intrahepatic transplantation limits their value; therefore, understanding the basis of MSCs survival and rejection may increase their utility. This study was aimed at determining the role of intrahepatic natural killer (NK) cells on MSCs survival and their retention in the liver shortly after transplant.
Procedures
Human MSCs were labeled with the Luc2-mKate2 dual-fusion reporter gene (MSCs-R), and the residence time and survival of MSCs-R xenografts after intrahepatic transplantation were evaluated by in vivo bioluminescence imaging (BLI). Coculture of MSCs and NK cells was performed to assess cytotoxicity. To evaluate the role of NK cells in rejection of the xenografted cells, the fates of transplanted MSCs-R were then assessed in vivo by BLI after activation of intrahepatic NK cells.
Results
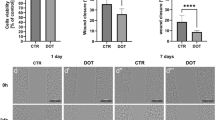
We observed a linear correlation between luciferase activity from live MSCs-R and cell number in vitro (R 2 = 0.9956). In vivo, we observed a gradual decline in bioluminescent signals from transplanted MSCs-R over a region corresponding to the liver in both the control group and the NK-activated group. However, the survival time and retention of intrahepatic MSCs-R decreased more rapidly in the NK-activated group of mice compared to the control group. This indicated that activated NK cells accelerate the elimination of transplanted MSCs. Also, we found that the number of hepatic NK cells and the expression of NK activation markers significantly increased after intrahepatic delivery of MSCs. This suggested that resident NK cells, in a resting state, were activated by intrahepatic transplantation of human MSCs. Taken together, the data suggests that activated hepatic NK cells mediate, in part, rejection of the MSCs xenografts. Cytotoxicity assays showed that activated NK cells may inhibit the proliferation of MSCs and, to a certain extent, induce MSCs death.
Conclusion
Human MSCs could be followed dynamically in vivo by BLI, and the role of murine hepatic NK cells, especially activated NK cells, could be inferred from the loss of signals from MSCs. This finding may have practical clinical implications in MSCs transplantation in treating liver disease.






Similar content being viewed by others
References
Sato Y, Araki H, Kato J et al (2005) Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 106:756–763
Oyagi S, Hirose M, Kojima M et al (2006) Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol 44:742–748
Kanazawa H, Fujimoto Y, Teratani T et al (2011) Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One 6:e19195
Yan Y, Xu W, Qian H et al (2009) Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int 29:356–365
Peng L, Xie DY, Lin BL et al (2011) Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 54:820–828
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822
Horwitz EM, Gordon PL, Koo WK et al (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A 99:8932–8937
Ryan JM, Barry FP, Murphy JM et al (2005) Mesenchymal stem cells avoid allogeneic rejection. J Inflamm 2:8
Koc ON, Day J, Nieder M et al (2002) Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy(MLD) and Hurler syndrome(MPS-IH). Bone Marrow Transplant 30:215–222
Arinzeh TL, Peter SJ, Archambault MP et al (2003) Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am 85-A:1927–1935
Fouillard L, Bensidhoum M, Bories D et al (2003) Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia 17:474–476
Cahill RA, Jones OY, Klemperer M et al (2004) Replacement of recipient stromal/mesenchymal cells after bone marrow transplantation using bone fragments and cultured osteoblast-like cells. Biol Blood Marrow Transplant 10:709–717
Ringdén O, Uzunel M, Rasmusson I et al (2006) Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81:1390–1397
Ringdén O, Uzunel M, Sundberg B et al (2007) Tissue repair using allogeneic mesenchymal stem cells for hemorrhagiccystitis, pneumome diastinum and perforated colon. Leukemia 21:2271–2276
Poncelet AJ, Vercruysse J, Saliez A et al (2007) Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 83:783–790
Coyne TM, Marcus AJ, Woodbury D et al (2006) Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells 24:2483–2492
Eliopoulos N, Stagg J, Lejeune L et al (2005) Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 106:4057–4065
Zangi L, Margalit R, Reich-Zeliger S et al (2009) Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 27:2865–2874
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Ranson T, Vosshenrich CA, Corcuff E et al (2003) IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A 100:2663–2668
Fehniger TA, Caligiuri MA (2001) Interleukin-15: biology and relevance to human disease. Blood 97:14–32
Vermijlen D, Luo D, Froelich CJ et al (2002) Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J Leukoc Biol 72:668–676
Ishiyama K, Ohdan H, Ohira M et al (2006) Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology 43:362–372
Ruggeri L, Capanni M, Urbani E et al (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295:2097–2100
Kean LS, Hamby K, Koehn B et al (2006) NK cells mediate costimulation blockade-resistant rejection of allogeneic stem cells during nonmyeloablative transplantation. Am J Transplant 6:292–304
Zhang B, Shan H, Li D et al (2012) The inhibitory effect of MSCs expressing TRAIL as a cellular delivery vehicle in combination with cisplatin on hepatocellular carcinoma. Cancer Biol Ther 13:1175–1184
Li Z, Hu X, Mao J et al (2015) Optimization of mesenchymal stem cells (MSCs) delivery dose and route in mice with acute liver injury by bioluminescence imaging. Mol Imaging Biol 17:185–194
Zhang J, Dong Z, Zhou R et al (2005) Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell Mol Immunol 2:271–280
Stoklasek TA, Schluns KS, Lefrançois L (2006) Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 177:6072–6080
Toma C, Wagner WR, Bowry S et al (2009) Fate of culture-expanded mesenchymal stem cells in the microvascula-ture: in vivo observations of cell kinetics. Circ Res 104:398–402
Spaggiari GM, Capobianco A, Becchetti S et al (2006) Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 107:1484–1490
Sotiropoulou PA, Perez SA, Gritzapis AD et al (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24:74–85
Gotherstrom C, Lundqvist A, Duprez IR et al (2011) Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy 13:269–278
Perez-Cunningham J, Ames E, Smith RC et al (2014) Natural killer cell subsets differentially reject embryonic stem cells based on licensing. Transplantation 97:992–998
Kroemer A, Xiao X, Degauque N et al (2008) The innate NK cells, allograft rejection, and a key role for IL-15. J Immunol 180:7818–7826
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. U1032002, 81430041, 81271621, 81071206, 81271562) and Key Clinical Research Project of Public Health Ministry of China 2010–2012 (No. 164).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Jj., Hu, Xj., Li, Zr. et al. In Vivo Bioluminescence Imaging of Transplanted Mesenchymal Stromal Cells and Their Rejection Mediated by Intrahepatic NK Cells. Mol Imaging Biol 19, 31–40 (2017). https://doi.org/10.1007/s11307-016-0962-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0962-9