Abstract
Purpose
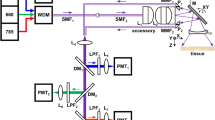
Early and effective detection of cancers of the gastrointestinal tract will require novel molecular probes and advances in instrumentation that can reveal functional changes in dysplastic and malignant tissues. Here, we describe adaptation of a wide-field clinical fiberscope to perform wide-field fluorescence imaging while preserving its white-light capability for the purpose of providing wide-field fluorescence imaging capability to point-of-care microscopes.
Procedures
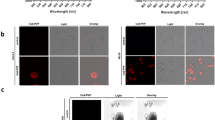
We developed and used a fluorescent fiberscope to detect signals from a quenched probe, BMV109, that becomes fluorescent when cleaved by, and covalently bound to, active cathepsin proteases. Cathepsins are expressed in inflammation- and tumor-associated macrophages as well as directly from tumor cells and are a promising target for cancer imaging. The fiberscope has a 1-mm outer diameter enabling validation via endoscopic exams in mice, and therefore we evaluated topically applied BMV109 for the ability to detect colon polyps in an azoxymethane-induced colon tumor model in mice.
Results
This wide-field endoscopic imaging device revealed consistent and clear fluorescence signals from BMV109 that specifically localized to the polypoid regions as opposed to the normal adjacent colon tissue (p < 0.004) in the murine colon carcinoma model.
Conclusions
The sensitivity of detection of BMV109 with the fluorescence fiberscope suggested utility of these tools for early detection at hard-to-reach sites. The fiberscope was designed to be used in conjunction with miniature, endoscope-compatible fluorescence microscopes for dual wide-field and microscopic cancer detection.





Similar content being viewed by others
References
American Cancer Society: Cancer Facts and Figures 2014. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014 (Accessed 17 Feb 2015).
(2011) Vital signs. Colorectal cancer screening, incidence, and mortality—United States, 2002-2010. In MMWR Morb Mortal Wkly Rep. pp 884-889.
Winawer SJ, Zauber AG, Ho MN et al (1993) Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 329:1977–1981
Zauber AG, Winawer SJ, O'Brien MJ et al (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366:687–696
Stallmach A, Schmidt C, Watson A, Kiesslich R (2011) An unmet medical need: advances in endoscopic imaging of colorectal neoplasia. J Biophotonics 4:482–489
Barrett T, Koyama Y, Hama Y et al (2007) In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res 13:6639–6648
Fujii T, Kamiya M, Urano Y (2014) In vivo imaging of intraperitoneally disseminated tumors in model mice by using activatable fluorescent small-molecular probes for activity of cathepsins. Bioconjug Chem 25:1838–1846
Kim SY, Myung SJ (2013) Optical molecular imaging for diagnosing intestinal diseases. Clin Endosc 46:620–626
Oh G, Yoo SW, Jung Y et al (2014) Intravital imaging of mouse colonic adenoma using MMP-based molecular probes with multi-channel fluorescence endoscopy. Biomed Opt Express 5:1677–1689
Urano Y, Asanuma D, Hama Y et al (2009) Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med 15:104–109
Verdoes M, Edgington LE, Scheeren FA et al (2012) A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem Biol 19:619–628
Verdoes M, Oresic Bender K, Segal E et al (2013) Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J Am Chem Soc 135:14726–14730
Gounaris E, Martin J, Ishihara Y et al (2013) Fluorescence endoscopy of cathepsin activity discriminates dysplasia from colitis. Inflamm Bowel Dis 19:1339–1345
Funovics MA, Alencar H, Su HS et al (2003) Miniaturized multichannel near infrared endoscope for mouse imaging. Mol Imaging 2:350–357
Funovics MA, Alencar H, Montet X et al (2006) Simultaneous fluorescence imaging of protease expression and vascularity during murine colonoscopy for colonic lesion characterization. Gastrointest Endosc 64:589–597
Miller SJ, Joshi BP, Feng Y et al (2011) In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe. PLoS One 6:e17384
Liu Z, Miller SJ, Joshi BP, Wang TD (2013) In vivo targeting of colonic dysplasia on fluorescence endoscopy with near-infrared octapeptide. Gut 62:395–403
Miller SJ, Lee CM, Joshi BP, Gaustad A, Seibel EJ, Wang TD (2012) Targeted detection of murine colonic dysplasia in vivo with flexible multispectral scanning fiber endoscopy. J Biomed Opt 17:021103
Becker C, Fantini MC, Neurath MF (2006) High resolution colonoscopy in live mice. Nat Protoc 1:2900–2904
Becker C, Fantini MC, Wirtz S et al (2005) In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 54:950–954
Ogihara T, Watanabe H, Namihisa A et al (1999) Clinical experience using a real time autofluorescence endoscopy system in the gastrointestinal tract. Diagn Ther Endosc 5:119–124
Mohamed MM, Sloane BF (2006) Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 6:764–775
Verdoes M, Florea BI, Menendez-Benito V et al (2006) A fluorescent broad-spectrum proteasome inhibitor for labeling proteasomes in vitro and in vivo. Chem Biol 13:1217–1226
Larsson M, Arvidsson S, Ekman C, Bayati A (2003) A model for chronic quantitative studies of colorectal sensitivity using balloon distension in conscious mice—effects of opioid receptor agonists. Neurogastroenterol Motil 15:371–381
Wang TD, Contag CH, Mandella MJ et al (2004) Confocal fluorescence microscope with dual-axis architecture and biaxial postobjective scanning. J Biomed Opt 9:735–742
Wang D, Chen Y, Leigh SY et al (2012) Microscopic delineation of medulloblastoma margins in a transgenic mouse model using a topically applied VEGFR-1 probe. Translat Oncol 5:408–414
Ra H, Piyawattanametha W, Gonzalez-Gonzalez E et al (2011) In vivo imaging of human and mouse skin with a handheld dual-axis confocal fluorescence microscope. J Invest Dermatol 131:1061–1066
Wang TD, Mandella MJ, Contag CH, Kino GS (2003) Dual-axis confocal microscope for high-resolution in vivo imaging. Opt Lett 28:414–416
Leggett B, Whitehall V (2010) Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 138:2088–2100
Nawa T, Kato J, Kawamoto H et al (2008) Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol 23:418–423
Okamoto M, Kawabe T, Yamaji Y et al (2005) Flat-type early colorectal cancer preferentially develops in right-sided colon in older patients. Dis Colon Rectum 48:101–107
Soetikno RM, Kaltenbach T, Rouse RV et al (2008) Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. J Am Med Assoc 299:1027–1035
Torlakovic E, Skovlund E, Snover DC et al (2003) Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 27:65–81
Diamond SJ, Enestvedt BK, Jiang Z et al (2011) Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc 74:135–140
Huang CS, O'Brien MJ, Yang S, Farraye FA (2004) Hyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathway. Am J Gastroenterol 99:2242–2255
Dacosta RS, Wilson BC, Marcon NE (2002) New optical technologies for earlier endoscopic diagnosis of premalignant gastrointestinal lesions. J Gastroenterol Hepatol 17(Suppl):S85–S104
DaCosta RS, Wilson BC, Marcon NE (2005) Optical techniques for the endoscopic detection of dysplastic colonic lesions. Curr Opin Gastroenterol 21:70–79
Inomata H, Tamai N, Aihara H et al (2013) Efficacy of a novel auto-fluorescence imaging system with computer-assisted color analysis for assessment of colorectal lesions. World J Gastroenterol 19:7146–7153
Singh R, Jayanna M, Navadgi S et al (2013) Narrow-band imaging with dual focus magnification in differentiating colorectal neoplasia. Dig Endosc 25(Suppl 2):16–20
Urquhart P, DaCosta R, Marcon N (2013) Endoscopic mucosal imaging of gastrointestinal neoplasia in 2013. Curr Gastroenterol Rep 15:330
Zavaleta CL, Garai E, Liu JT et al (2013) A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc Natl Acad Sci U S A 110:E2288–E2297
Muguruma N, Miyamoto H, Okahisa T, Takayama T (2013) Endoscopic molecular imaging: status and future perspective. Clin Endosc 46:603–610
Fisher LR, Hasler WL (2012) New vision in video capsule endoscopy: current status and future directions. Nat Rev Gastroenterol Hepatol 9:392–405
Chu X, Poh CK, Li L et al (2010) Epitomized summarization of wireless capsule endoscopic videos for efficient visualization. Med Image Comput Comput Assist Interv 13:522–529
Hsiung PL, Hardy J, Friedland S et al (2008) Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med 14:454–458
Uddin MJ, Crews BC, Blobaum AL et al (2010) Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. Cancer Res 70:3618–3627
Segal E, Prestwood TR, van der Linden WA et al (2015) Detection of intestinal cancer by local, topical application of a quenched fluorescence probe for cysteine cathepsins. Chem Biol 22:148–158
Goetz M, Ziebart A, Foersch S et al (2010) In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology 138:435–446
Ginty F, Adak S, Can A et al (2008) The relative distribution of membranous and cytoplasmic met is a prognostic indicator in stage I and II colon cancer. Clin Cancer Res 14:3814–3822
Wielenga VJ, van der Voort R, Taher TE et al (2000) Expression of c-Met and heparan-sulfate proteoglycan forms of CD44 in colorectal cancer. Am J Pathol 157:1563–1573
Ellis LM, Takahashi Y, Liu W, Shaheen RM (2000) Vascular endothelial growth factor in human colon cancer: biology and therapeutic implications. Oncologist 5(Suppl 1):11–15
Togashi K, Hewett DG, Whitaker DA, Hume GE, Francis L, Appleyard MN (2006) The use of acetic acid in magnification chromocolonoscopy for pit pattern analysis of small polyps. Endoscopy 38:613–616
Acknowledgments
The authors thank the Stanford Small Animal Imaging Facility for resources and technical support. We would also like to thank Pankaj Pasricha, Martijn Verdoes, Laura Edgington, James Amos-Landgraff, Laura Bronsart, Bonnie King, and the laboratory of Lawrence Marnett for equipment, assistance, and discussions aiding in the development of our imaging system. This work was supported in part by the National Cancer Institute (U54 CA136465 and P50 CA114747), the Canary Foundation, and a generous gift from the Chambers Family Foundation for Excellence in Pediatric Research. Steven Sensarn was supported by the Stanford Cancer Imaging Training fellowship from the NCI (5 T32 CA 9695-19). Cristina Zavaleta was supported by the National Cancer Institute of the National Institutes of Health under Award Number K22 CA160834.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Steven Sensarn and Cristina L. Zavaleta contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sensarn, S., Zavaleta, C.L., Segal, E. et al. A Clinical Wide-Field Fluorescence Endoscopic Device for Molecular Imaging Demonstrating Cathepsin Protease Activity in Colon Cancer. Mol Imaging Biol 18, 820–829 (2016). https://doi.org/10.1007/s11307-016-0956-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0956-7