Abstract
Purpose
The purpose of this study was to characterize imaging biomarkers for the potential benefit of hypoxia-inducible factor-1 (HIF-1)α inhibition (by PX-12) during 5-fluorouracil (5-FU) chemotherapy in the treatment of colorectal cancer (CRC).
Procedures
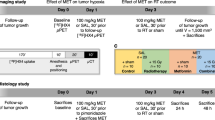
Therapy response to 5-FU ± PX-12 was assessed with baseline [18F]fluoromisonidazole ([18F]FMISO) and longitudinal 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) positron emission computed tomography (μPET/CT) in CRC xenograft model (n = 36) during breathing of a hypoxic (10 % O2) or normoxic (21 % O2) atmosphere. Ex vivo, immunohistochemistry was performed.
Results
Baseline [18F]FMISO uptake and relative tumor volume (RTV) 2 days after 5-FU or 5-FU + PX-12 administration correlated significantly (p ≤ 0.01). Under hypoxic breathing conditions, [18F]FDG uptake (−53.1 ± 8.4 %) and Ki67 expression (−16 %) decreased and RTV stagnated in the 5-FU + PX-12 treatment group, but not in 5-FU alone-treated tumors. Under normoxic breathing, [18F]FDG uptake (−23.5 ± 15.2 % and −72.8 ± 7.1 %) and Ki67 expression (−5 % and −19 %) decreased and RTV stagnated in both the 5-FU and the combination treatment group, respectively.
Conclusion
Baseline [18F]FMISO μPET may predict the beneficial effect of HIF-1α inhibition during 5-FU chemotherapy in CRC.






Similar content being viewed by others
References
Nagaraju GP, Bramhachari PV, Raghu G, El-Rayes BF (2015) Hypoxia inducible factor-1α: its role in colorectal carcinogenesis and metastasis. Cancer Lett 366:11–18
Vaupel P, Mayer A (2007) Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 26:225–239
Bertout JA, Patel SA, Simon MC (2008) The impact of O2 availability on human cancer. Nat Rev Cancer 8:967–975
Ruan K, Song G, Ouyang G (2009) Role of hypoxia in the hallmarks of human cancer. J Cell Biochem 107:1053–1062
Ravizza R, Molteni R, Gariboldi MB et al (2009) Effect of HIF-1 modulation on the response of two- and three-dimensional cultures of human colon cancer cells to 5-fluorouracil. Eur J Cancer 45:890–898
Gustavsson B, Carlsson G, Machover D et al (2015) A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer 14:1–10
Semenza GL (2006) Development of novel therapeutic strategies that target HIF-1. Expert Opin Ther Targets 10:267–280
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732
Krohn KA, Link JM, Mason RP (2008) Molecular imaging of hypoxia. J Nucl Med 49:129S–148S
Rajendran JG, Krohn KA (2015) F-18 fluoromisonidazole for imaging tumor hypoxia: imaging the microenvironment for personalized cancer therapy. Sem Nucl Med 45:151–162
Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2:683–693
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Kim J-W, Gao P, Dang CV (2007) Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev 26:291–298
Denko NC (2008) Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 8:705–713
Wijsman R, Kaanders JHAM, Oyen WJG, Bussink J (2013) Hypoxia and tumor metabolism in radiation oncology: targets visualized by positron emission tomography. Q J Nucl Med Mol Imaging 57:244–256
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Bentzen L, Keiding S, Horsman MR et al (2000) Feasibility of detecting hypoxia in experimental mouse tumours with 18F-fluorinated tracers and positron emission tomography. Acta Oncol 39:629–637
Mees G, Dierckx R, Vangestel C et al (2013) Pharmacologic activation of tumor hypoxia: a means to increase tumor 2-deoxy-2-[18F]fluoro-D-glucose uptake? Mol Imaging 12:49–58
Powis G, Kirkpatrick DL (2007) Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol 7:392–397
Welsh SJ, Williams RR, Birmingham A et al (2003) The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther 2:235–243
Kim YH, Coon A, Baker AF, Powis G (2010) Antitumor agent PX-12 inhibits HIF-1α protein levels through an Nrf2/PMF-1-mediated increase in spermidine/spermine acetyl transferase. Cancer Chemother Pharmacol 68:405–413
Wouters A, Pauwels B, Lambrechts HAJ et al (2009) Chemoradiation interactions under reduced oxygen conditions: cellular characteristics of an in vitro model. Cancer Lett 286:180–188
Adamsen TCH, Grierson JR, Krohn KA (2005) A new synthesis of the labeling precursor for [18F]-fluoromisonidazole. J Label Compd Radiopharm 48:923–927
De Bruycker S, Vangestel C, Verbrugghen T et al (2014) Baseline [18F]FMISO and early [18F]FDG μPET changes as imaging biomarkers for HIF-1α inhibition combined with 5-FU in colorectal cancer [abstract]. European Molecular Imaging Meeting
Dubois L, Lieuwes NG, Janssen MH et al (2011) Preclinical evaluation and validation of [18F]HX4, a promising hypoxia marker for PET imaging. Proc Natl Acad Sci U S A 108:14620–14625
Babsky AM, Zhang H, Hekmatyar SK et al (2007) Monitoring chemotherapeutic response in RIF-1 tumors by single-quantum and triple-quantum-filtered 23Na MRI, 1H diffusion-weighted MRI and PET imaging. Magn Reson Imaging 25:1015–1023
Barthel H, Cleij MC, Collingridge DR et al (2003) 3“-deoxy-3-”[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res 63:3791–3798
Kamm YJL, Peters GJ, Hull WE et al (2003) Correlation between 5-fluorouracil metabolism and treatment response in two variants of C26 murine colon carcinoma. Br J Cancer 89:754–762
Choudhury KR, Yagle KJ, Swanson PE et al (2010) A robust automated measure of average antibody staining in immunohistochemistry images. J Histochem Cytochem 58:95–107
Beck R, Roper B, Carlsen JM et al (2007) Pretreatment 18F-FAZA PET predicts success of hypoxia-directed radiochemotherapy using tirapazamine. J Nucl Med 48:973–980
Wyss MT, Honer M, Schubiger PA, Ametamey SM (2005) NanoPET imaging of [18F]fluoromisonidazole uptake in experimental mouse tumours. Eur J Nucl Med Mol Imaging 33:311–318
Troost EGC, Laverman P, Kaanders JHAM et al (2006) Imaging hypoxia after oxygenation-modification: comparing [18F]FMISO autoradiography with pimonidazole immunohistochemistry in human xenograft tumors. Radiother Oncol 80:157–164
Campanile C, Arlt MJE, Kramer SD et al (2013) Characterization of different osteosarcoma phenotypes by PET imaging in preclinical animal models. J Nucl Med 54:1362–1368
Busk M, Horsman MR, Kristjansen PEG et al (2008) Aerobic glycolysis in cancers: implications for the usability of oxygen-responsive genes and fluorodeoxyglucose-PET as markers of tissue hypoxia. Int J Cancer 122:2726–2734
Rajendran JG, Mankoff DA, O'Sullivan F et al (2004) Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 10:2245–2252
Jordan BF, Runquist M, Raghunand N et al (2005) The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res 11:529–536
Ramanathan RK, Kirkpatrick DL, Belani CP et al (2007) A phase I pharmacokinetic and pharmacodynamic study of PX-12, a novel inhibitor of thioredoxin-1, in patients with advanced solid tumors. Clin Cancer Res 13:2109–2114
Dupuis NP, Kusumoto T, Robinson MF et al (1995) Restoration of tumor oxygenation after cytotoxic therapy by a perflubron emulsion/carbogen breathing. Artif Cells Blood Substit Immobil Biotechnol 23:423–429
Grassetto G, Capirci C, Marzola MC et al (2011) Colorectal cancer: prognostic role of 18F-FDG-PET/CT. Abdom Imaging 37:575–579
Keen H, Pichler B, Kukuk D et al (2011) An evaluation of 2-deoxy-2-[18F]-fluoro-D-glucose and 3′-deoxy-3′-[18F]-fluorothymidine uptake in human tumor xenograft models. Mol Imaging Biol 14:355–365
Sharma RI, Smith TAD (2008) Colorectal tumor cells treated with 5-FU, oxaliplatin, irinotecan, and cetuximab exhibit changes in 18F-FDG incorporation corresponding to hexokinase activity and glucose transport. J Nucl Med 49:1386–1394
Acknowledgments
We gratefully acknowledge Philippe Joye and Caroline Berghmans of the Molecular Imaging Center Antwerp (MICA) and Christophe Hermans of the Center for Oncological Research (CORE) for their excellent technical assistance, and Thomas Verbrugghen of MICA for the [18F]FMISO productions. This work was funded by the University of Antwerp, Belgium through a PhD grant for Sven De Bruycker, an associate professor position for Patrick Pauwels and Steven Staelens, and a full professor position for Sigrid Stroobants; by Antwerp University Hospital, Belgium, through a postdoctoral position for Christel Vangestel (Innovative Medicines Initiative, Quic-Concept), a full-time position for Leonie wyffels, and a departmental position for Tim Van den Wyngaert, Patrick Pauwels, and Sigrid Stroobants; and by the Research Foundation Flanders, Belgium (FWO Vlaanderen) through a postdoctoral fellowship for An Wouters.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
(PDF 198 kb)
Rights and permissions
About this article
Cite this article
De Bruycker, S., Vangestel, C., Van den Wyngaert, T. et al. Baseline [18F]FMISO μPET as a Predictive Biomarker for Response to HIF-1α Inhibition Combined with 5-FU Chemotherapy in a Human Colorectal Cancer Xenograft Model. Mol Imaging Biol 18, 606–616 (2016). https://doi.org/10.1007/s11307-015-0926-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0926-5