Abstract
Purpose
Disrupted-in-schizophrenia-1 (DISC1) is a promising genetic susceptibility factor for major psychiatric conditions, such as schizophrenia. We hypothesized that the mutant DISC1 alters the homeostasis of multi-receptor interactions between dopaminergic [dopamine 2/3 (D2/3R)], glutamatergic [metabotropic glutamate 5 (mGluR5)], cannabinoid 1 (CB1R), and nicotinic acetylcholine (α4β2-nAChR) receptors in the brains of mice with inducible forebrain neuronal expression of dominant-negative mutant DISC1.
Procedures
The quantitative in vitro autoradiography was performed with positron emission tomography (PET) ligands using [11C]raclopride (D2/3R), [11C]ABP688 (mGluR5), [11C]OMAR (CB1R), and [18F]AZAN (nAChR). Total binding (pmol/cc) from standard and binding index, defined as [(region of interest − reference) / reference], was analyzed in the parasagittal sections. The cerebellum was used as a reference for D2/3R, mGluR5, and α4β2-nAChR, while the midbrain was the reference tissue for CB1R, because of the high density of CB1R in the cerebellum.
Results
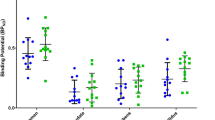
We observed a significant positive correlation between mGluR5 and D2/3R in the nucleus accumbens (NAc) in mutant DISC1 (rho = 0.6, p = 0.04; y = 0.02 x + 6.7) and a trend of negative correlation between those receptors in the dorsal striatum (DS) in control animals (rho = −0.5, p = 0.09; y = −0.03 x + 23), suggesting a co-release of dopamine (DA) and glutamate (Glu) in the NAc, but not in the DS. There were trends of an inverse relationship between striatal CB1R and D2/3R (rho = −0.7, p = 0.07) as well as between dorsal thalamic nAChR and striatal D2/3R (rho = −0.5, p = 0.08). There was no statistically significant difference of the individual receptor density in the majority of brain regions.
Conclusions
The mutant DISC1 altered the homeostasis of multi-receptor interactions of coincident signaling of DA and Glu in the NAc, but not in the DS, and mutually negative control of striatal CB1R and D2/3R. Multi-receptor mapping with PET ligands in relevant animal models could be a valuable translational approach for psychiatric drug development.






Similar content being viewed by others
Abbreviations
- BI:
-
Binding index
- CB1R:
-
Cannabinoid 1 receptor
- DISC1:
-
Disrupted-in-schizophrenia-1
- D2/3R:
-
Dopamine 2/3 receptor
- DLU:
-
Digital light unit
- DS:
-
Dorsal striatum
- GABA:
-
Gamma amino butyric acid
- K d :
-
Dissociation constant
- mGluR5 :
-
Metabotropic glutamate receptor 5
- NAc:
-
Nucleus accumbens
- nAChR:
-
Nicotinic acetylcholine receptor
- PET:
-
Positron emission tomography
- PFC:
-
Prefrontal cortex
- ROI:
-
Region of interest
- SN:
-
Substantia nigra
- TB:
-
Total binding
- SCZ:
-
Schizophrenia
- VGLUT2:
-
Vesicular glutamate transporter 2
- VMAT2:
-
Vesicular monoamine transporter 2
- VTA:
-
Ventral tegmental area
- VS:
-
Ventral striatum
References
Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169
Millar JK, Christie S, Anderson S et al (2001) Genomic structure and localisation within a linkage hotspot of disrupted in schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol Psychiatry 6:173–178
Chubb JE, Bradshaw NJ, Soares DC et al (2008) The DISC locus in psychiatric illness. Mol Psychiatry 13:36–64
Pletnikov MV, Ayhan Y, Nikolskaia O et al (2008) Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry 13:173–186
Sawa A, Snyder SH (2002) Schizophrenia: diverse approaches to a complex disease. Science 296:692–695
Dal Bo G, St-Gelais F, Danik M et al (2004) Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem 88:1398–1405
Joyce MP, Rayport S (2000) Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience 99(3):445–456
Chuhma N, Choi WY, Mingote S et al (2009) Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience 164:1068–1083
Hnasko TS, Chuhma N, Zhang H et al (2010) Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65:643–656
Stuber GD, Hnasko TS, Britt JP et al (2010) Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci 30:8229–8233
Houchi H, Babovic D, Pierrefiche O et al (2005) CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology 30:339–349
Thanos PK, Gopez V, Delis F et al (2011) Upregulation of cannabinoid type 1 receptors in dopamine D2 receptor knockout mice is reversed by chronic forced ethanol consumption. Alcohol Clin Exp Res 35:19–27
Mansvelder HD, McGehee DS (2002) Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol 53:606–617
Strome EM, Jivan S, Doudet DJ (2005) Quantitative in vitro phosphor imaging using [3H] and [18F] radioligands: the effects of chronic desipramine treatment on serotonin 5-HT2 receptors. J Neurosci Methods 141:143–154
Farde L, Ehrin E, Eriksson L et al (1985) Substituted benzamides as ligands for visualization of dopamine receptor binding in the human brain by positron emission tomography. Proc Natl Acad Sci U S A 82:3863–3867
Ametamey SM, Kessler LJ, Honer M et al (2006) Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med 47:698–705
Horti AG, Fan H, Kuwabara H et al (2006) 11C-JHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J Nucl Med 47:1689–1696
Horti AG, Gao Y, Kuwabara H et al (2010) Development of radioligands with optimized imaging properties for quantification of nicotinic acetylcholine receptors by positron emission tomography. Life Sci 86:575–584
Farde L, Hall H, Ehrin E, Sedvall G (1986) Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 231:258–261
Wong DF, Wagner HN Jr, Tune LE et al (1986) Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science 234:1558–1563
Laurelle M, Kegeles L, Abi-Dargham A (2003) Glutamate, dopamine, and schizophrenia from pathophysiology to treatment. Ann N Y Acad Sci 1003:138–158
Jaaro-Peled H, Niwa M, Foss CA et al (2013) Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Hum Mol Genet 22:1574–1580
Kim SJ, Sullivan JM, Wang S et al (2014) Voxelwise lp-ntPET for detecting localized, transient dopamine release of unknown timing: sensitivity analysis and application to cigarette smoking in the PET scanner. Hum Brain Mapp 35:4876–4891
Niwa M, Jaaro-Peled H, Tankou S et al (2013) Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 339:335–339
Hayashi-Takagi A, Takaki M, Graziane N et al (2010) Disrupted-in-schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci 13:327–332
O’Donnell P, Grace AA (1995) Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15:3622–3639
Corti C, Xuereb JH, Crepaldi L et al (2011) Altered levels of glutamatergic receptors and Na(+)/K(+) ATPase-alpha1 in the prefrontal cortex of subjects with schizophrenia. Schizophr Res 128:7–14
Tokunaga M, Seneca N, Shin RM et al (2009) Neuroimaging and physiological evidence for involvement of glutamatergic transmission in regulation of the striatal dopaminergic system. J Neurosci 29:1887–1896
Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 24(511):421–427
Ishikawa M, Okata M, Huang YH et al (2013) Dopamine triggers heterosynaptic plasticity. J Neurosci 33:6759–6765
Saito A, Ballinger MD, Pletnikov MV et al (2013) Endocannabinoid system: potential novel targets for treatment of schizophrenia. Neurobiol Dis 53:10–17
Pickel VM, Chan J, Kearn CS, Mackie K (2006) Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol 495:299–313
Mackie K (2005) Cannabinoid receptor homo- and heterodimerization. Life Sci 77:1667–1673
Martin AB, Fernandez-Espejo E, Ferrer B et al (2008) Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology 33:1667–1679
Moldrich G, Wenger T (2000) Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides 21:1735–1742
Marcellino D, Carriba P, Filip M et al (2008) Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 54:815–823
Wong DF, Kuwabara H, Kim J et al (2013) PET imaging of high-affinity α4β2 nicotinic acetylcholine receptors in humans with [18F]AZAN, a radioligand with optimal brain kinetics. J Nucl Med 54:1308–1448
Breese CR, Lee MJ, Adams CE et al (2000) Abnormal regulation of high affinity nicotine receptors in subjects with schizophrenia. Neuropsychopharmacology 23:351–364
Robinson TE, Becker JB (1985) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev 11:157–198
Malmberg A, Jerning E, Mohell N (1996) Critical reevaluation of spiperone and benzamide binding to dopamine D2 receptors: evidence for identical binding sites. Eur J Pharmacol 303:123–128
Farde L, Eriksson L, Blomquist G et al (1989) Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET–a comparison to the equilibrium analysis. J Cereb Blood Flow Metab 9:696–708
Acknowledgments
The study was supported by ARRA RO1NIMH (MVP), NARSAD (MVP, AK), NH094268 (MVP, AK), and NIH (NIBIB, NIDA, and NIAAA) Training grant for Clinician Scientists in Imaging Research (5T32EB006351-05) (JK), NIDA 5R33DA016182-05 (W.B.M). The authors would like to thank Drs Adjmal Nahimi (Aarhus University, Aarhus, Denmark) and Albert H. Gjedde (University of Copenhagen, Denmark) for the protocol for autoradiography and Emily Gean for her assistance with the preparation of this manuscript.
Author Contribution
Contributing to conception and design: JK, MVP, and DFW; acquiring data: HV, AH, WBM, DPH, HTR, and RFD; analyzing and interpreting data: JK, LZ, and BZ; drafting the manuscript: JK, MVP, and AK; enhancing intellectual content: VP, JRB, and AK; approving the final content of the manuscript: MVP and DFW.
Conflict of Interest
The authors declare they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 379 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Horti, A.G., Mathews, W.B. et al. Quantitative Multi-modal Brain Autoradiography of Glutamatergic, Dopaminergic, Cannabinoid, and Nicotinic Receptors in Mutant Disrupted-In-Schizophrenia-1 (DISC1) Mice. Mol Imaging Biol 17, 355–363 (2015). https://doi.org/10.1007/s11307-014-0786-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0786-4