Abstract
Purpose
Molecular imaging has the potential to provide quantitative information about specific biological aspects of developing atherosclerotic lesions. This requires the generation of reliable, highly specific plaque tracers. This study reports a new camelid single-domain antibody fragment (sdAb) targeting the Lectin-like oxidized low-density lipoprotein receptor (LOX-1), a biomarker for the detection and molecular phenotyping of vulnerable atherosclerotic plaques.
Procedures
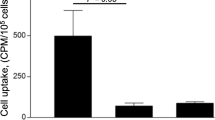
A camelid sdAb was generated and selected for high affinity binding to LOX-1. Ex vivo biodistribution and in vivo single photon emission computed tomography (SPECT)/computed tomography (CT) imaging studies were performed in wild-type mice and in fat-fed atherosclerotic apolipoprotein E-deficient mice with 99mTc-labeled sdAbs. Gamma-counting and autoradiography analyses were performed on dissected aorta segments with different degrees of plaque burden. The specificity of the LOX-1-targeting sdAb was evaluated by blocking with unlabeled sdAb or by comparison with a nontargeting 99mTc-labeled control sdAb.
Results
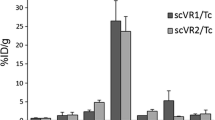
We generated a sdAb binding LOX-1 with a KD of 280 pM ± 62 pM affinity. After 99mTc-labeling, the tracer had radiochemical purity higher then 99 % and retained specificity in in vitro binding studies. Tracer blood clearance was fast with concomitant high kidney retention. At 3 h after injection, uptake in tissues other than plaques was low and not different than background, suggesting a restricted expression pattern of LOX-1. Conversely, uptake in aortic segments increased with plaque content and was due to specific LOX-1 binding. In vivo SPECT/CT imaging 160 min after injection in atherosclerotic mice confirmed specific targeting of LOX-1-expressing aortic plaques.
Conclusions
The LOX-sdAb specifically targets LOX-1-expressing atherosclerotic plaques within hours after injection. The possibility to image LOX-1 rapidly after administration combined with the favourable biodistribution of a sdAb are beneficial for molecular phenotyping of atherosclerotic plaques and the generation of a future prognostic tracer.






Similar content being viewed by others
References
Langer HF, Haubner R, Pichler BJ, Gawaz M (2008) Radionuclide imaging: a molecular key to the atherosclerotic plaque. J Am Coll Cardiol 52:1–12
Joshi NV, Vesey AT, Williams MC et al (2013) F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 383:705–713
Matter CM, Stuber M, Nahrendorf M (2009) Imaging of the unstable plaque: how far have we got? Eur Heart J 30:2566–2574
Sinusas AJ, Thomas JD, Mills G (2011) The future of molecular imaging. JACC Cardiovasc Imaging 4:799–806
Fleg JL, Stone GW, Fayad ZA et al (2012) Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging 5:941–955
Sawamura T, Kume N, Aoyama T et al (1997) An endothelial receptor for oxidized low-density lipoprotein. Nature 386:73–77
Kataoka H, Kume N, Miyamoto S et al (1999) Expression of lectin like oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 99:3110–3117
Li DY, Chen HJ, Staples ED et al (2002) Oxidized low-density lipoprotein receptor LOX-1 and apoptosis in human atherosclerotic lesions. J Cardiovasc Pharmacol Ther 7:147–153
Saito A, Fujimura M, Inoue T et al (2010) Relationship between lectin-like oxidized low-density lipoprotein receptor 1 expression and preoperative echogenic findings of vulnerable carotid plaque. Acta Neurochir (Wien) 152:589–595
Ishino S, Mukai T, Kume N et al (2007) Lectin-like oxidized LDL receptor-1 (LOX-1) expression is associated with atherosclerotic plaque instability—analysis in hypercholesterolemic rabbits. Atherosclerosis 195:48–56
Chen J, Li D, Schaefer R, Mehta JL (2006) Cross-talk between dyslipidemia and renin-angiotensin system and the role of LOX-1 and MAPK in atherogenesis studies with the combined use of rosuvastatin and candesartan. Atherosclerosis 184:295–301
Mehta JL, Sanada N, Hu CP et al (2007) Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100:1634–1642
White SJ, Sala-Newby GB, Newby AC (2011) Overexpression of scavenger receptor LOX-1 in endothelial cells promotes atherogenesis in the ApoE(−/−) mouse model. Cardiovasc Pathol 20:369–373
Li D, Patel AR, Klibanov AL et al (2010) Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging 3:464–472
Ishino S, Mukai T, Kuge Y et al (2008) Targeting of lectin like oxidized low-density lipoprotein receptor 1 (LOX-1) with 99mTc-labeled anti-LOX-1 antibody: potential agent for imaging of vulnerable plaque. J Nucl Med 49:1677–1685
Wen S, Liu DF, Cui Y et al (2013) In vivo MRI detection of carotid atherosclerotic lesions and kidney inflammation in ApoE-deficient mice by using LOX-1 targeted iron nanoparticles. Nanomedicine
De Vos J, Devoogdt N, Lahoutte T, Muyldermans S (2013) Camelid single-domain antibody-fragment engineering for (pre)clinical in vivo molecular imaging applications: adjusting the bullet to its target. Expert Opin Biol Ther 13:1149–1160
Hamers-Casterman C, Atarhouch T, Muyldermans S et al (1993) Naturally occurring antibodies devoid of light chains. Nature 363:446–448
Arbabi Ghahroudi M, Desmyter A et al (1997) Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett 414:521–526
Devoogdt N, Xavier C, Hernot S et al (2012) Molecular imaging using nanobodies: a case study. Methods Mol Biol 911:559–567
Vaneycken I, D’Huyvetter M, Hernot S et al (2011) Immuno-imaging using nanobodies. Curr Opin Biotechnol 22:877–881
Xavier C, Vaneycken I, D'Huyvetter M et al (2013) Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-Anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med 54:776–784
Gainkam LO, Huang L, Caveliers V et al (2008) Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. J Nucl Med 49:788–795
Oliveira S, van Dongen GA, Stigter-van Walsum M et al (2012) Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody. Mol Imaging 11:33–46
Hernot S, Unnikrishnan S, Du Z et al (2012) Nanobody-coupled microbubbles as novel molecular tracer. J Control Release 158:346–353
Xavier C, Devoogdt N, Hernot S et al (2012) Site-specific labeling of his-tagged nanobodies with 99mTc: a practical guide. Methods Mol Biol 911:485–490
Broisat A, Hernot S, Toczek J et al (2012) Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ Res 110:927–937
Conrath KE, Lauwereys M, Galleni M et al (2001) Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the Camelidae. Antimicrob Agents Chemother 45:2807–2812
Vaneycken I, Govaert J, Vincke C et al (2010) In vitro analysis and in vivo tumor targeting of a humanized, grafted nanobody in mice using pinhole SPECT/micro-CT. J Nucl Med 51:1099–1106
Nakashima Y, Plump AS, Raines EW et al (1994) ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 14:133–140
Vaneycken I, Devoogdt N, Van Gassen N et al (2011) Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J 25:2433–2446
Pirillo A, Norata GD, Catapano AL (2013) LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm 2013:152786
Kuge Y, Kume N, Ishino S et al (2008) Prominent lectin-like oxidized low density lipoprotein (LDL) receptor-1 (LOX-1) expression in atherosclerotic lesions is associated with tissue factor expression and apoptosis in hypercholesterolemic rabbits. Biol Pharm Bull 31:1475–1482
Smith DD, Tan X, Raveendran VV et al (2012) Mast cell deficiency attenuates progression of atherosclerosis and hepatic steatosis in apolipoprotein E-null mice. Am J Physiol Heart Circ Physiol 302:H2612–H2621
Fisker Hag AM, Pedersen SF, Kjaer A (2008) Gene expression of LOX-1, VCAM-1, and ICAM-1 in pre-atherosclerotic mice. Biochem Biophys Res Commun 377:689–693
Meir KS, Leitersdorf E (2004) Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol 24:1006–1014
Movahedi K, Schoonooghe S, Laoui D et al (2012) Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res 72:4165–4177
Tchouate Gainkam LO, Caveliers V, Devoogdt N et al (2011) Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice. Contrast Media Mol Imaging 6:85–92
Vaneycken I, Xavier C, Blykers A et al (2011) Synthesis and first in vivo evaluation of 18F-anti-HER2-nanobodies: a new probe for PET imaging of HER2 expression in breast cancer. J Nucl Med 52:664–664
Acknowledgments
We thank Cindy Peleman, Isabel Remory and Emmy De Blay for their technical assistance. Jens De Vos and Sam Massa have a PhD fellowship of the Research Foundation Flanders (FWO). Tony Lahoutte is a Senior Clinical Investigator of the Research Foundation Flanders (FWO). The research at ICMI is funded by the Belgian State, Nationaal Kankerplan, Vlaamse liga tegen kanker, Stichting tegen kanker, FWO-Vlaanderen, IWT and Vrije Universiteit Brussel.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3555 kb)
Rights and permissions
About this article
Cite this article
De Vos, J., Mathijs, I., Xavier, C. et al. Specific Targeting of Atherosclerotic Plaques in ApoE−/− Mice Using a New Camelid sdAb Binding the Vulnerable Plaque Marker LOX-1. Mol Imaging Biol 16, 690–698 (2014). https://doi.org/10.1007/s11307-014-0731-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0731-6