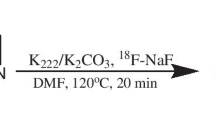Abstract
Purpose
The aim of the present study was to develop short half-lived tools for in vitro and in vivo β-amyloid imaging in mice, for which no suitable PET tracers are available.
Procedures
Five 13N-labelled azo compounds (1–5) were synthesized using a three-step process using cyclotron-produced [13N]NO3 −. Biodistribution studies were performed using positron emission tomography–computed tomography (PET–CT) on 20-month-old healthy, wild-type (WT) mice. In vivo and in vitro binding assays were performed using PET-CT and autoradiography, respectively, on 20-month-old healthy (WT) mice and transgenic (Tg2576) Alzheimer's disease model mice.
Results
13N-labelled azo compounds were prepared with decay corrected radiochemical yields in the range 27 ± 4 % to 39 ± 4 %. Biodistribution studies showed good blood–brain barrier penetration for compounds 1 and 3–5; good clearance data were also obtained for compounds 1–3 and 5. Compounds 2, 3 and 5 (but not 1) showed a significant uptake in β-amyloid-rich structures when assayed in in vitro autoradiographic studies. PET studies showed significant uptake of compounds 2 and 3 in the cortex of transgenic animals that exhibit β-amyloid deposits.
Conclusions
The results underscore the potential of compounds 2 and 3 as in vitro and in vivo markers for β-amyloid in animal models of Alzheimer's disease.






Similar content being viewed by others
References
Selkoe DJ (2012) Preventing Alzheimer's disease. Science 337:1488–1492
Sajid J, Elhaddaoui A, Turrell S (1997) Investigation of the binding of Congo red to amyloid in Alzheimer's diseased tissue. J Mol Struct 408–409:181–184
Hardy JA, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: problems and progress on the road to therapeutics. Science 297:353–356
Klunk WE, Engler H, Nordberg A et al (2004) Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 55:306–319
Vandenberghe R, Van Laere K, Ivanoiu A et al (2010) 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 68:319–329
Thurfjell L, Lötjönen J, Lundqvist R et al (2012) Combination of biomarkers: PET [18F]flutemetamol imaging and structural MRI in dementia and mild cognitive impairment. Neurodegener Dis 10:246–249
Cselényi Z, Jönhagen ME, Forsberg A et al (2012) Clinical validation of 18F-AZD4694, an amyloid-β-specific PET radioligand. J Nucl Med 53:415–424
Rowe CC, Ackerman U, Browne W et al (2008) Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 7:129–135
Wong DF, Rosenberg PB, Zhou Y et al (2010) In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18-AV-45 (florbetapir [corrected] F 18). J Nucl Med 51:913–920
Mathis CA, Mason NS, Lopresti BJ, Klunk WE (2012) Development of positron emission tomography β-amyloid plaque imaging agents. Semin Nucl Med 42:423–432
Kepe V, Moghbel MC, Långström B et al (2013) Amyloid-β positron emission tomography imaging probes: a critical review. J Alzheimers Dis. doi:10.3233/JAD-130485
Montine TJ, Phelps CH, Beach TG et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11
Hyman BT, Phelps CH, Beach TG et al (2012) Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13
Agdeppa ED, Kepe V, Liu J et al (2001) Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer's disease. J Neurosci 21:RC189
Kuntner C, Kesner AL, Bauer M et al (2009) Limitations of small animal PET imaging with [18F]FDDNP and FDG for quantitative studies in a transgenic mouse model of Alzheimer’s disease. Mol Imaging Biol 11:236–240
Toyama H, Ye D, Ichise M et al (2005) PET imaging of brain with the beta-amyloid probe, [11C]6-OH-BTA-1, in a transgenic mouse model of Alzheimer’s disease. Eur J Nucl Med Mol Imaging 32:593–600
Klunk WE, Lopresti BJ, Ikonomovic MD et al (2005) Binding of the positron emission tomography tracer Pittsburgh Compound-B reflects the amount of amyloid-β in Alzheimer’s disease brain but not in transgenic mouse brain. J Neurosci 25:10598–10606
Higuchi M, Maeda J, Ji B et al (2010) In vivo visualization of key molecular processes involved in Alzheimer’s disease pathogenesis: insights from neuroimaging research in humans and rodent models. Biochim Biophys Acta 1802:373–388
Maeda J, Ji B, Irie T et al (2007) Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J Neurosci 27:10957–10968
Poisnel G, Dhilly M, Moustié O et al (2012) PET imaging with [18F]AV-45 in an APP/PS1-21 murine model of amyloid plaque deposition. Neurobiol Aging 33:2561–2571
Gómez-Vallejo V, Borrell JI, Llop J (2010) A convenient synthesis of 13N-labelled azo compounds: a new route for the preparation of amyloid imaging PET probes. Eur J Med Chem 45:5318–5323
Gaja V, Gómez-Vallejo V, Cuadrado-Tejedor M et al (2012) Synthesis of 13N-labelled radiotracers by using microfluidic technology. J Labelled Comp Radiopharm 55:332–338
Logan J, Fowler JS, Volkow ND et al (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840
Reilly JF, Games D, Rydel RE et al (2003) Amyloid deposition in the hippocampus and entorhinal cortex: quantitative analysis of a transgenic mouse model. Proc Natl Acad Sci U S A 100:4837–4842
Dickson DW, Farlo J, Davies P et al (1988) Alzheimer’s disease. A double-labelling immunohistochemical study of senile plaques. Am J Pathol 132:86–101
Villemagne VL, Klunk WE, Mathis CA et al (2012) Aβ imaging: feasible, pertinent, and vital to progress in Alzheimer's disease. Eur J Nucl Med Mol Imaging 39:209–219
Fodero-Tavoletti MT, Okamura N, Furumoto S et al (2011) 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 134:1089–1100
Zhang W, Arteaga J, Cashion DK et al (2012) A highly selective and specific PET tracer for imaging of tau pathologies. J Alzheimers Dis 31:601–602
Xia C-F, Arteaga J, Chen G, et al. (2013) [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement, in press, Corrected Proof, doi: 10.1016/j.jalz.2012.11.008
Manook A, Yousefi BH, Willuweit A et al (2012) Small-Animal PET imaging of Amyloid-beta plaques with [11C]PIB and its multi-modal validation in an APP/PS1 mouse model of Alzheimer’s disease. PLoS ONE 7(3):e31310
Dishino DD, Welch MJ, Kilbourn MR, Raichle ME (1983) Relationship between lipophilicity and brain extraction of C-11-labeled radiopharmaceuticals. J Nucl Med 24:1030–1038
Acknowledgments
This work was supported by EU grant PITN-GA-2012-316882 and by intramural FIMA (Fundación para la Investigación Médica Aplicada) funds. We acknowledge Dr. Juan Domingo Gispert for fruitful discussion about experimental design details.
Conflict of interest
None of the authors has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaja, V., Gómez-Vallejo, V., Puigivila, M. et al. Synthesis and Evaluation of 13N-Labelled Azo Compounds for β-Amyloid Imaging in Mice. Mol Imaging Biol 16, 538–549 (2014). https://doi.org/10.1007/s11307-013-0708-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0708-x