Abstract
Introduction
Dairy cows experience metabolic stress during the transition from late pregnancy to early lactation, due to the complex adaptation processes affecting energy homeostasis in support of milk production, collectively referred to as homeorhesis. According to the individual efficiency of this adaptation, some cows develop severe metabolic diseases while others are able to maintain metabolic health.
Objectives
This study aimed to characterize patterns and changes of metabolic phenotype during the transition period, and to identify how far different metabolic pathways are affected by or contributing to the complex system of homeorhesis.
Methods
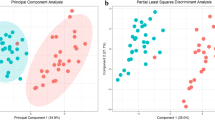
Blood samples were collected from 26 German Holstein cows, repeatedly during the transition period: 42 and 10 days before calving and 3, 21 and 100 days after calving. Blood serum samples were subjected to a liquid chromatography–mass spectrometry based targeted metabolomics analysis using the AbsoluteIDQ p180 Kit of Biocrates Life Science AG (Innsbruck, Austria). Processed metabolomics data were evaluated by multivariate data analysis techniques such as principal component analysis (PCA) and partial least squares-discriminant analysis and by heatmap visualization.
Results
The PCA revealed a clear separation according to sampling days, indicating a notable shift of the metabolic phenotype during the transition period. The heatmap showed that acylcarnitines provided a consistent clustering within sampling days, while the concentration of glycerophospholipids and sphingolipids were remarkably decreased 10 days before and 3 days after calving than earlier and later in the transition period.
Conclusion
Analyzing longitudinal changes of the blood metabolome and identifying new biomarkers by this approach can help understanding the multifaceted metabolic adaptation of transition dairy cows.






Similar content being viewed by others
References
Adams, S. H., Hoppel, C. L., Lok, K. H., Zhao, L., Wong, S. W., Minkler, P. E., et al. (2009). Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid -oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. Journal of Nutrition, 139(6), 1073–1081. doi:10.3945/jn.108.103754.
Ametaj, B. N. (2015, May, 8–10). A systems veterinary approach in understanding transition cow diseases: Metabolomics. In: Proceedings of the 4th international symposium on dairy cow nutrition and milk quality, session 1, advances in fundamental research, Beijing (pp. 78–85). May 8–10.
Başoğlu, A., Başpinar, N., & Coşkun, A. (2014). NMR-based metabolomic evaluation in dairy cows with displaced abomasum. Turkish Journal of Veterinary and Animal Sciences, 38, 325–330. doi:10.3906/vet-1310-52.
Bauman, D. E., & Currie, B. W. (1980). Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. Journal of Dairy Science, 63(9), 1514–1529. doi:10.3168/jds.S0022-0302(80)83111-0.
Bradford, B. J., Yuan, K., & Ylioja, C. (2016). Managing complexity: Dealing with systemic crosstalk in bovine physiology1. Journal of Dairy Science, 99(6), 4983–4996. doi:10.3168/jds.2015-10271.
Butler, W. R. (2000). Nutritional interactions with reproductive performance in dairy cattle. Animal Reproduction Science, 60, 449–457.
Butler, W. R., & Smith, R. D. (1989). interrelationships between energy balance and postpartum reproductive function in dairy cattle. Journal of Dairy Science, 72(3), 767–783. doi:10.3168/jds.S0022-0302(89)79169-4.
De Koster, J. D., & Opsomer, G. (2013). Insulin resistance in dairy cows. Veterinary Clinics of North America: Food Animal Practice, 29(2), 299–322. doi:10.1016/j.cvfa.2013.04.002.
Drackley, J. K. (1999). Biology of dairy cows during the transition period: The final frontier? Journal of Dairy Science, 82(11), 2259–2273. doi:10.3168/jds.S0022-0302(99)75474-3.
Drackley, J. K., Overton, T. R., & Douglas, G. N. (2001). Adaptations of glucose and long-chain fatty acid metabolism in liver of dairy cows during the periparturient period. Journal of Dairy Science, 84, E100–E112. doi:10.3168/jds.S0022-0302(01)70204-4.
Fischer, H. P. (2008). Mathematical modeling of complex biological systems: From parts lists to understanding systems behavior. Alcohol Research & Health, 31(1), 49.
Gault, C. R., Obeid, L. M., & Hannun, Y. A. (2010). An overview of sphingolipid metabolism: From synthesis to breakdown. In Sphingolipids as Signaling and Regulatory Molecules (pp. 1–23). New York: Springer. Accessed May 3, 2016 from http://link.springer.com/chapter/10.1007/978-1-4419-6741-1_1
Geary, U., Lopez-Villalobos, N., Begley, N., McCoy, F., O’Brien, B., O’Grady, L., et al. (2012). Estimating the effect of mastitis on the profitability of Irish dairy farms. Journal of Dairy Science, 95(7), 3662–3673. doi:10.3168/jds.2011-4863.
Goff, J. P., & Horst, R. L. (1997). Physiological changes at parturition and their relationship to metabolic disorders. Journal of Dairy Science, 80(7), 1260–1268. doi:10.3168/jds.S0022-0302(97)76055-7.
Ha, C. Y., Kim, J. Y., Paik, J. K., Kim, O. Y., Paik, Y.-H., Lee, E. J., et al. (2012). The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 diabetes: Metabolic intermediates in patients with T2DM. Clinical Endocrinology, 76(5), 674–682. doi:10.1111/j.1365-2265.2011.04244.x.
Hailemariam, D., Mandal, R., Saleem, F., Dunn, S. M., Wishart, D. S., & Ametaj, B. N. (2014a). Identification of predictive biomarkers of disease state in transition dairy cows. Journal of Dairy Science, 97(5), 2680–2693. doi:10.3168/jds.2013-6803.
Hailemariam, D., Mandal, R., Saleem, F., Dunn, S. M., Wishart, D. S., & Ametaj, B. N. (2014b). Metabolomics approach reveals altered plasma amino acid and sphingolipid profiles associated with patholological state in transition dairy cows. Current Metabolomics, 2(3), 184–195.
Huber, K., Dänicke, S., Rehage, J., Sauerwein, H., Otto, W., Rolle-Kampczyk, U., et al. (2016). Metabotypes with properly functioning mitochondria and anti-inflammation predict extended productive life span in dairy cows. Scientific Reports, 6, 24642. doi:10.1038/srep24642.
Huber, K., Kenez, A., McNamara, J. P., & Shields, S. L. (2014). A systems approach to determine the effect of changes in gene expression in adipose tissue on productive and reproductive efficiency in dairy cattle. Animal Production Science, 54, 1224–1227. doi:10.1071/AN14209.
Hume, D. A., Whitelaw, C. B. A., & Archibald, A. L. (2011). The future of animal production: Improving productivity and sustainability. The Journal of Agricultural Science, 149(S1), 9–16. doi:10.1017/S0021859610001188.
Imhasly, S., Bieli, C., Naegeli, H., Nyström, L., Ruetten, M., & Gerspach, C. (2015). Blood plasma lipidome profile of dairy cows during the transition period. BMC Veterinary Research. doi:10.1186/s12917-015-0565-8.
Imhasly, S., Naegeli, H., Baumann, S., von Bergen, M., Luch, A., Jungnickel, H., et al. (2014). Metabolomic biomarkers correlating with hepatic lipidosis in dairy cows. BMC Veterinary Research, 10(1), 1.
Inchaisri, C., Jorritsma, R., Vos, P. L. A. M., van der Weijden, G. C., & Hogeveen, H. (2010). Economic consequences of reproductive performance in dairy cattle. Theriogenology, 74(5), 835–846. doi:10.1016/j.theriogenology.2010.04.008.
Ingvartsen, K. L. (2006). Feeding- and management-related diseases in the transition cow. Animal Feed Science and Technology, 126(3–4), 175–213. doi:10.1016/j.anifeedsci.2005.08.003.
Ingvartsen, K. L., Dewhurst, R. J., & Friggens, N. C. (2003). On the relationship between lactational performance and health: Is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Livestock Production Science, 83(2), 277–308.
Ingvartsen, K. L., & Friggens, N. C. (2005). To what extent do variabilities in hormones, metabolites and energy intake explain variability in milk yield? Domestic Animal Endocrinology, 29(2), 294–304. doi:10.1016/j.domaniend.2005.05.001.
Ingvartsen, K. L., & Moyes, K. (2013). Nutrition, immune function and health of dairy cattle. Animal, 7(s1), 112–122. doi:10.1017/S175173111200170X.
Kossaibati, M. A., & Esslemont, R. J. (1997). The costs of production diseases in dairy herds in England. The Veterinary Journal, 154(1), 41–51. doi:10.1016/S1090-0233(05)80007-3.
LeBlanc, S. (2010). Monitoring metabolic health of dairy cattle in the transition period. Journal of Reproduction and Development, 56(S), S29–S35. doi:10.1262/jrd.1056S29.
Li, P., Yin, Y.-L., Li, D., Woo Kim, S., & Wu, G. (2007). Amino acids and immune function. British Journal of Nutrition, 98(2), 237. doi:10.1017/S000711450769936X.
Loor, J. J., Bertoni, G., Hosseini, A., Roche, J. R., & Trevisi, E. (2013). Functional welfare—using biochemical and molecular technologies to understand better the welfare state of peripartal dairy cattle. Animal Production Science. doi:10.1071/AN12344.
Loor, J. J., Vailati-Riboni, M., McCann, J. C., Zhou, Z., & Bionaz, M. (2015). Triennial lactation symposium: Nutrigenomics in livestock: Systems biology meets nutrition. Journal of Animal Science, 93(12), 5554–5574.
Maeda, Y., Ohtsuka, H., & Oikawa, M. (2012). Effect of the periparturient period on blood free amino acid concentration in dairy cows/healthy cows. Journal of Veterinary Medicine and Animal Health, 4(9), 124–129.
McCarthy, M. M., Mann, S., Nydam, D. V., Overton, T. R., & McArt, J. A. A. (2015). Short communication: Concentrations of nonesterified fatty acids and β-hydroxybutyrate in dairy cows are not well correlated during the transition period. Journal of Dairy Science, 98(9), 6284–6290. doi:10.3168/jds.2015-9446.
McNamara, J. P. (2012). Ruminant nutrition symposium: A systems approach to integrating genetics, nutrition, and metabolic efficiency in dairy cattle. Journal of Animal Science, 90(6), 1846–1854. doi:10.2527/jas.2011-4609.
McNamara, J. P. (2015). Triennial lactation symposium: Systems biology of regulatory mechanisms of nutrient metabolism in lactation. Journal of Animal Science, 93(12), 5575–5585.
Mulligan, F. J., & Doherty, M. L. (2008). Production diseases of the transition cow. The Veterinary Journal, 176(1), 3–9. doi:10.1016/j.tvjl.2007.12.018.
Oltenacu, P. A., & Broom, D. M. (2010). The impact of genetic selection for increased milk yield on the welfare of dairy cows. Animal Welfare, 19(1), 39–49.
Ospina, P. A., McArt, J. A., Overton, T. R., Stokol, T., & Nydam, D. V. (2013). Using nonesterified fatty acids and β-hydroxybutyrate concentrations during the transition period for herd-level monitoring of increased risk of disease and decreased reproductive and milking performance. The Veterinary Clinics of North America. Food Animal Practice, 29(2), 387–412. doi:10.1016/j.cvfa.2013.04.003.
Reid, I. M., Roberts, C. J., Treacher, R. J., & Williams, L. A. (1986). Effect of body condition at calving on tissue mobilization, development of fatty liver and blood chemistry of dairy cows. Animal Production, 43(1), 7–15. doi:10.1017/S0003356100018298.
Rico, J. E., Bandaru, V. V. R., Dorskind, J. M., Haughey, N. J., & McFadden, J. W. (2015). Plasma ceramides are elevated in overweight Holstein dairy cows experiencing greater lipolysis and insulin resistance during the transition from late pregnancy to early lactation. Journal of Dairy Science, 98(11), 7757–7770. doi:10.3168/jds.2015-9519.
RStudio Team. (2015). RStudio: Integrated development for R. Boston: RStudio, Inc. http://www.rstudio.com/
Sordillo, L. M., & Aitken, S. L. (2009). Impact of oxidative stress on the health and immune function of dairy cattle. Veterinary Immunology and Immunopathology, 128(1–3), 104–109. doi:10.1016/j.vetimm.2008.10.305.
Sun, L. W., Zhang, H. Y., Wu, L., Shu, S., Xia, C., Xu, C., et al. (2014). 1H-Nuclear magnetic resonance-based plasma metabolic profiling of dairy cows with clinical and subclinical ketosis. Journal of Dairy Science, 97(3), 1552–1562. doi:10.3168/jds.2013-6757.
Tienken, R., Kersten, S., Frahm, J., Meyer, U., Locher, L., Rehage, J., et al. (2015). Effects of an energy-dense diet and nicotinic acid supplementation on production and metabolic variables of primiparous or multiparous cows in periparturient period. Archives of Animal Nutrition, 69(5), 319–339. doi:10.1080/1745039X.2015.1073002.
Trevisi, E., Amadori, M., Riva, F., Bertoni, G., & Bani, P. (2014). Evaluation of innate immune responses in bovine forestomachs. Research in Veterinary Science, 96(1), 69–78. doi:10.1016/j.rvsc.2013.11.011.
Vernon, R. G. (2005). Lipid metabolism during lactation: A review of adipose tissue–liver interactions and the development of fatty liver. The Journal of Dairy Research, 72(4), 460–469. doi:10.1017/S0022029905001299.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Research, 43(W1), W251–W257. doi:10.1093/nar/gkv380.
Yea, K., Kim, J., Yoon, J. H., Kwon, T., Kim, J. H., Lee, B. D., et al. (2009). Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. Journal of Biological Chemistry, 284(49), 33833–33840. doi:10.1074/jbc.M109.024869.
Zhang, H., Wu, L., Xu, C., Xia, C., Sun, L., & Shu, S. (2013). Plasma metabolomic profiling of dairy cows affected with ketosis using gas chromatography/mass spectrometry. BMC Veterinary Research, 9(1), 1.
Zhu, C., Liang, Q., Hu, P., Wang, Y., & Luo, G. (2011). Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta, 85(4), 1711–1720. doi:10.1016/j.talanta.2011.05.036.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the German Research Foundation (DFG, Bonn, Germany; Grant number DA 558/6-1).
Conflict of interest
Ákos Kenéz, Sven Dänicke, Ulrike Rolle-Kampczyk, Martin von Bergen, Korinna Huber declares that they have no conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kenéz, Á., Dänicke, S., Rolle-Kampczyk, U. et al. A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics 12, 165 (2016). https://doi.org/10.1007/s11306-016-1112-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1112-8