Abstract
Introduction
Chlamydia trachomatis (Ct), is the leading cause of sexually transmitted infections worldwide. Host transcriptomic- or proteomic profiling studies have identified key molecules involved in establishment of Ct infection or the generation of anti Ct-immunity. However, the contribution of the host metabolome is not known.
Objectives
The objective of this study was to determine the contribution of host metabolites in genital Ct infection.
Methods
We used high-performance liquid chromatography-mass spectrometry, and mapped lipid profiles in genital swabs obtained from female guinea pigs at days 3, 9, 15, 30 and 65 post Ct serovar D intravaginal infection.
Results
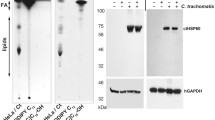
Across all time points assessed, 13 distinct lipid species including choline, ethanolamine and glycerol were detected. Amongst these metabolites, phosphatidylcholine (PC) was the predominant phospholipid detected from animals actively shedding bacteria i.e., at 3, 9, and 15 days post infection. However, at days 30 and 65 when the animals had cleared the infection, PC was observed to be decreased compared to previous time points. Mass spectrometry analyses of PC produced in guinea pigs (in vivo) and 104C1 guinea pig cell line (in vitro) revealed distinct PC species following Ct D infection. Amongst these, PC 16:0/18:1 was significantly upregulated following Ct D infection (p < 0.05, >twofold change) in vivo and in vitro infection models investigated in this report. Exogenous addition of PC 16:0/18:1 resulted in significant increase in Ct D in Hela 229 cells.
Conclusion
This study demonstrates a role for host metabolite, PC 16:0/18:1 in regulating genital Ct infection in vivo and in vitro.



Similar content being viewed by others
References
Adekoya, N., Truman, B., Landen, M., & Centers for Disease, C., & Prevention. (2015). Incidence of notifiable diseases among American Indians/Alaska Natives—United States, 2007–2011. Morbidity and Mortality Weekly Report, 64(1), 16–19.
Arkatkar, T., Gupta, R., Li, W., Yu, J. J., Wali, S., Neal Guentzel, M., et al. (2015). Murine MicroRNA-214 regulates intracellular adhesion molecule (ICAM1) gene expression in genital Chlamydia muridarum infection. Immunology, 145(4), 534–542. doi:10.1111/imm.12470.
Banhart, S., Saied, E. M., Martini, A., Koch, S., Aeberhard, L., Madela, K., et al. (2014). Improved plaque assay identifies a novel anti-Chlamydia ceramide derivative with altered intracellular localization. Antimicrobial Agents and Chemotherapy, 58(9), 5537–5546. doi:10.1128/AAC.03457-14.
Brotman, R. M., Ravel, J., Bavoil, P. M., Gravitt, P. E., & Ghanem, K. G. (2014). Microbiome, sex hormones, and immune responses in the reproductive tract: Challenges for vaccine development against sexually transmitted infections. Vaccine, 32(14), 1543–1552. doi:10.1016/j.vaccine.2013.10.010.
Brunham, R. C., & Rappuoli, R. (2013). Chlamydia trachomatis control requires a vaccine. Vaccine, 31(15), 1892–1897. doi:10.1016/j.vaccine.2013.01.024.
Capmany, A., & Damiani, M. T. (2010). Chlamydia trachomatis intercepts Golgi-derived sphingolipids through a Rab14-mediated transport required for bacterial development and replication. PLoS ONE, 5(11), e14084. doi:10.1371/journal.pone.0014084.
Carlson, J. H., Whitmire, W. M., Crane, D. D., Wicke, L., Virtaneva, K., Sturdevant, D. E., et al. (2008). The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infection and Immunity, 76(6), 2273–2283. doi:10.1128/IAI.00102-08.
Champion, C. I., Kickhoefer, V. A., Liu, G., Moniz, R. J., Freed, A. S., Bergmann, L. L., et al. (2009). A vault nanoparticle vaccine induces protective mucosal immunity. PLoS ONE, 4(4), e5409. doi:10.1371/journal.pone.0005409.
Cole, L. K., Dolinsky, V. W., Dyck, J. R., & Vance, D. E. (2011). Impaired phosphatidylcholine biosynthesis reduces atherosclerosis and prevents lipotoxic cardiac dysfunction in ApoE −/− Mice. Circulation Research, 108(6), 686–694. doi:10.1161/CIRCRESAHA.110.238691.
Cole, L. K., Vance, J. E., & Vance, D. E. (2012). Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochimica et Biophysica Acta, 1821(5), 754–761. doi:10.1016/j.bbalip.2011.09.009.
Cruz-Fisher, M. I., Cheng, C., Sun, G., Pal, S., Teng, A., Molina, D. M., et al. (2011). Identification of immunodominant antigens by probing a whole Chlamydia trachomatis open reading frame proteome microarray using sera from immunized mice. Infection and Immunity, 79(1), 246–257. doi:10.1128/IAI.00626-10.
Cunningham, K., Stansfield, S. H., Patel, P., Menon, S., Kienzle, V., Allan, J. A., et al. (2013). The IL-6 response to Chlamydia from primary reproductive epithelial cells is highly variable and may be involved in differential susceptibility to the immunopathological consequences of chlamydial infection. BMC Immunol, 14, 50. doi:10.1186/1471-2172-14-50.
Darville, T., & Hiltke, T. J. (2010). Pathogenesis of genital tract disease due to Chlamydia trachomatis. Journal of Infectious Diseases, 201(Suppl 2), S114–S125.
de Alwis, N. M., & Day, C. P. (2008). Non-alcoholic fatty liver disease: The mist gradually clears. Journal of Hepatology, 48(Suppl 1), S104–S112. doi:10.1016/j.jhep.2008.01.009.
De Clercq, E., Kalmar, I., & Vanrompay, D. (2013). Animal models for studying female genital tract infection with Chlamydia trachomatis. Infection and Immunity, 81(9), 3060–3067. doi:10.1128/IAI.00357-13.
de Jonge, M. I., Keizer, S. A., El Moussaoui, H. M., van Dorsten, L., Azzawi, R., van Zuilekom, H. I., et al. (2011). A novel guinea pig model of Chlamydia trachomatis genital tract infection. Vaccine, 29(35), 5994–6001. doi:10.1016/j.vaccine.2011.06.037.
Derre, I. (2015). Chlamydiae interaction with the endoplasmic reticulum: Contact, function and consequences. Cellular Microbiology, 17(7), 959–966. doi:10.1111/cmi.12455.
Derre, I., Swiss, R., & Agaisse, H. (2011). The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathogens, 7(6), e1002092. doi:10.1371/journal.ppat.1002092.
Derrick, T., Roberts, C., Rajasekhar, M., Burr, S. E., Joof, H., Makalo, P., et al. (2013). Conjunctival MicroRNA expression in inflammatory trachomatous scarring. PLoS Neglected Tropical Diseases, 7(3), e2117. doi:10.1371/journal.pntd.0002117.
Deruaz, M., & Luster, A. D. (2015). Chemokine-mediated immune responses in the female genital tract mucosa. Immunology and Cell Biology, 93(4), 347–354. doi:10.1038/icb.2015.20.
Doria, M. L., Cotrim, Z., Macedo, B., Simoes, C., Domingues, P., Helguero, L., et al. (2012). Lipidomic approach to identify patterns in phospholipid profiles and define class differences in mammary epithelial and breast cancer cells. Breast Cancer Research and Treatment, 133(2), 635–648. doi:10.1007/s10549-011-1823-5.
Elwell, C. A., & Engel, J. N. (2012). Lipid acquisition by intracellular Chlamydiae. Cellular Microbiology, 14(7), 1010–1018. doi:10.1111/j.1462-5822.2012.01794.x.
Elwell, C. A., Jiang, S., Kim, J. H., Lee, A., Wittmann, T., Hanada, K., et al. (2011). Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathogens, 7(9), e1002198. doi:10.1371/journal.ppat.1002198.
Fahy, E., Subramaniam, S., Murphy, R. C., Nishijima, M., Raetz, C. R., Shimizu, T., et al. (2009). Update of the LIPID MAPS comprehensive classification system for lipids. Journal of Lipid Research, 50(Suppl), S9–S14. doi:10.1194/jlr.R800095-JLR200.
Ferreira, R., Borges, V., Nunes, A., Borrego, M. J., & Gomes, J. P. (2013). Assessment of the load and transcriptional dynamics of Chlamydia trachomatis plasmid according to strains’ tissue tropism. Microbiological Research, 168(6), 333–339. doi:10.1016/j.micres.2013.02.001.
Furuse, Y., Finethy, R., Saka, H. A., Xet-Mull, A. M., Sisk, D. M., Smith, K. L., et al. (2014). Search for microRNAs expressed by intracellular bacterial pathogens in infected mammalian cells. PLoS ONE, 9(9), e106434. doi:10.1371/journal.pone.0106434.
Gao, X., Zhang, Q., Meng, D., Isaac, G., Zhao, R., Fillmore, T. L., et al. (2012). A reversed-phase capillary ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) method for comprehensive top-down/bottom-up lipid profiling. Analytical and Bioanalytical Chemistry, 402(9), 2923–2933. doi:10.1007/s00216-012-5773-5.
Gondek, D. C., Olive, A. J., Stary, G., & Starnbach, M. N. (2012). CD4 + T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J Immunol, 189(5), 2441–2449. doi:10.4049/jimmunol.1103032.
Gottlieb, S. L., Low, N., Newman, L. M., Bolan, G., Kamb, M., & Broutet, N. (2014). Toward global prevention of sexually transmitted infections (STIs): The need for STI vaccines. Vaccine, 32(14), 1527–1535. doi:10.1016/j.vaccine.2013.07.087.
Gross, R. W., & Han, X. (2011). Lipidomics at the interface of structure and function in systems biology. Chemistry & Biology, 18(3), 284–291. doi:10.1016/j.chembiol.2011.01.014.
Gupta, R., Arkatkar, T., Yu, J. J., Wali, S., Haskins, W. E., Chambers, J. P., et al. (2015). Chlamydia muridarum infection associated host MicroRNAs in the murine genital tract and contribution to generation of host immune response. American Journal of Reproductive Immunology, 73(2), 126–140. doi:10.1111/aji.12281.
Hackstadt, T., Rockey, D. D., Heinzen, R. A., & Scidmore, M. A. (1996). Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO Journal, 15(5), 964–977.
Hackstadt, T., Scidmore, M. A., & Rockey, D. D. (1995). Lipid metabolism in Chlamydia trachomatis-infected cells: Directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proceedings of the National Academy of Sciences USA, 92(11), 4877–4881.
Hafner, L. M. (2015). Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception, 92(2), 108–115. doi:10.1016/j.contraception.2015.01.004.
Hafner, L., Beagley, K., & Timms, P. (2008). Chlamydia trachomatis infection: Host immune responses and potential vaccines. Mucosal Immunology, 1(2), 116–130. doi:10.1038/mi.2007.19.
Hafner, L. M., Wilson, D. P., & Timms, P. (2014). Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine, 32(14), 1563–1571. doi:10.1016/j.vaccine.2013.08.020.
Hatch, G. M., & McClarty, G. (2004). C. trachomatis-infection accelerates metabolism of phosphatidylcholine derived from low density lipoprotein but does not affect phosphatidylcholine secretion from hepatocytes. BMC Microbiology, 4, 8. doi:10.1186/1471-2180-4-8.
He, Q., Takizawa, Y., Hayasaka, T., Masaki, N., Kusama, Y., Su, J., et al. (2014). Increased phosphatidylcholine (16:0/16:0) in the folliculus lymphaticus of Warthin tumor. Analytical and Bioanalytical Chemistry, 406(24), 5815–5825. doi:10.1007/s00216-014-7890-9.
Huston, W. M., Harvie, M., Mittal, A., Timms, P., & Beagley, K. W. (2012). Vaccination to protect against infection of the female reproductive tract. Expert Review of Clinical Immunology, 8(1), 81–94. doi:10.1586/eci.11.80.
Igietseme, J. U., Omosun, Y., Partin, J., Goldstein, J., He, Q., Joseph, K., et al. (2013). Prevention of Chlamydia-induced infertility by inhibition of local caspase activity. Journal of Infectious Diseases, 207(7), 1095–1104. doi:10.1093/infdis/jit009.
Igietseme, J. U., Uriri, I. M., Kumar, S. N., Ananaba, G. A., Ojior, O. O., Momodu, I. A., et al. (1998). Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infection and Immunity, 66(9), 4030–4035.
Ishikawa, S., Tateya, I., Hayasaka, T., Masaki, N., Takizawa, Y., Ohno, S., et al. (2012). Increased expression of phosphatidylcholine (16:0/18:1) and (16:0/18:2) in thyroid papillary cancer. PLoS ONE, 7(11), e48873. doi:10.1371/journal.pone.0048873.
Jiang, J., Karimi, O., Ouburg, S., Champion, C. I., Khurana, A., Liu, G., et al. (2012). Interruption of CXCL13-CXCR5 axis increases upper genital tract pathology and activation of NKT cells following chlamydial genital infection. PLoS ONE, 7(11), e47487. doi:10.1371/journal.pone.0047487.
Jupelli, M., Murthy, A. K., Chaganty, B. K., Guentzel, M. N., Selby, D. M., Vasquez, M. M., et al. (2011). Neonatal chlamydial pneumonia induces altered respiratory structure and function lasting into adult life. Laboratory Investigation, 91(10), 1530–1539. doi:10.1038/labinvest.2011.103.
Karunakaran, K. P., Yu, H., Jiang, X., Chan, Q., Moon, K. M., Foster, L. J., et al. (2015). Outer membrane proteins preferentially load MHC class II peptides: Implications for a Chlamydia trachomatis T cell vaccine. Vaccine, 33(18), 2159–2166. doi:10.1016/j.vaccine.2015.02.055.
Kokes, M., Dunn, J. D., Granek, J. A., Nguyen, B. D., Barker, J. R., Valdivia, R. H., et al. (2015). Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host & Microbe, 17(5), 716–725. doi:10.1016/j.chom.2015.03.014.
Lal, J. A., Malogajski, J., Verweij, S. P., de Boer, P., Ambrosino, E., Brand, A., et al. (2013). Chlamydia trachomatis infections and subfertility: Opportunities to translate host pathogen genomic data into public health. Public Health Genomics, 16(1–2), 50–61. doi:10.1159/000346207.
Li, S., Goins, B., Hrycushko, B. A., Phillips, W. T., & Bao, A. (2012a). Feasibility of eradication of breast cancer cells remaining in postlumpectomy cavity and draining lymph nodes following intracavitary injection of radioactive immunoliposomes. Molecular Pharmaceutics, 9(9), 2513–2522. doi:10.1021/mp300132f.
Li, S., Goins, B., Zhang, L., & Bao, A. (2012b). Novel multifunctional theranostic liposome drug delivery system: Construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imaging. Bioconjugate Chemistry, 23(6), 1322–1332. doi:10.1021/bc300175d.
Li, W., Murthy, A. K., Guentzel, M. N., Seshu, J., Forsthuber, T. G., Zhong, G., et al. (2008). Antigen-specific CD4 + T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. Journal of Immunology, 180(5), 3375–3382.
Li, W., Murthy, A. K., Lanka, G. K., Chetty, S. L., Yu, J. J., Chambers, J. P., et al. (2013). A T cell epitope-based vaccine protects against chlamydial infection in HLA-DR4 transgenic mice. Vaccine, 31(48), 5722–5728. doi:10.1016/j.vaccine.2013.09.036.
Lyons, J. M., Ouburg, S., & Morre, S. A. (2009). An integrated approach to Chlamydia trachomatis infection: The ICTI Consortium, an update. Drugs of Today, 45 Suppl B, 15–23.
Mascellino, M. T., Boccia, P., & Oliva, A. (2011). Immunopathogenesis in Chlamydia trachomatis infected women. ISRN Obstetrics and Gynecol, 2011, 436936. doi:10.5402/2011/436936.
Mfuh, A. M., Mahindaratne, M. P., Quintero, M. V., Lakner, F. J., Bao, A., Goins, B. A., et al. (2011). Novel asparagine-derived lipid enhances distearoylphosphatidylcholine bilayer resistance to acidic conditions. Langmuir, 27(8), 4447–4455. doi:10.1021/la105085k.
Mirrashidi, K. M., Elwell, C. A., Verschueren, E., Johnson, J. R., Frando, A., Von Dollen, J., et al. (2015). Global mapping of the inc-human interactome reveals that retromer restricts chlamydia infection. Cell Host & Microbe, 18(1), 109–121. doi:10.1016/j.chom.2015.06.004.
Moore, E. R., Fischer, E. R., Mead, D. J., & Hackstadt, T. (2008). The chlamydial inclusion preferentially intercepts basolaterally directed sphingomyelin-containing exocytic vacuoles. Traffic, 9(12), 2130–2140. doi:10.1111/j.1600-0854.2008.00828.x.
Morrison, R. P., & Caldwell, H. D. (2002). Immunity to murine chlamydial genital infection. Infection and Immunity, 70(6), 2741–2751.
Muller, C., Dietz, I., Tziotis, D., Moritz, F., Rupp, J., & Schmitt-Kopplin, P. (2013). Molecular cartography in acute Chlamydia pneumoniae infections–a non-targeted metabolomics approach. Analytical and Bioanalytical Chemistry, 405(15), 5119–5131. doi:10.1007/s00216-013-6732-5.
Murthy, A. K., Li, W., Chaganty, B. K., Kamalakaran, S., Guentzel, M. N., Seshu, J., et al. (2011). Tumor necrosis factor alpha production from CD8 + T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infection and Immunity, 79(7), 2928–2935. doi:10.1128/IAI.05022-11.
Neuendorf, E., Gajer, P., Bowlin, A. K., Marques, P. X., Ma, B., Yang, H., et al. (2015). Chlamydia caviae infection alters abundance but not composition of the guinea pig vaginal microbiota. Pathogens and Disease, 73(4), ftv019. doi:10.1093/femspd/ftv019.
Nogueira, C. V., Zhang, X., Giovannone, N., Sennott, E. L., & Starnbach, M. N. (2015). Protective immunity against Chlamydia trachomatis can engage both CD4 + and CD8 + T cells and bridge the respiratory and genital mucosae. Journal of Immunology, 194(5), 2319–2329. doi:10.4049/jimmunol.1402675.
Oberley, R. E., Ault, K. A., Neff, T. L., Khubchandani, K. R., Crouch, E. C., & Snyder, J. M. (2004a). Surfactant proteins A and D enhance the phagocytosis of Chlamydia into THP-1 cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 287(2), L296–L306. doi:10.1152/ajplung.00440.2003.
Oberley, R. E., Goss, K. L., Ault, K. A., Crouch, E. C., & Snyder, J. M. (2004b). Surfactant protein D is present in the human female reproductive tract and inhibits Chlamydia trachomatis infection. Molecular Human Reproduction, 10(12), 861–870. doi:10.1093/molehr/gah117.
Owusu-Edusei, K, Jr, Chesson, H. W., Gift, T. L., Brunham, R. C., & Bolan, G. (2015). Cost-effectiveness of Chlamydia vaccination programs for young women. Emerging Infectious Diseases, 21(6), 960–968. doi:10.3201/eid2106.141270.
Owusu-Edusei, K, Jr, Chesson, H. W., Gift, T. L., Tao, G., Mahajan, R., Ocfemia, M. C., et al. (2013). The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sexually Transmitted Diseases, 40(3), 197–201. doi:10.1097/OLQ.0b013e318285c6d2.
Ozolins, D., D’Elios, M. M., Lowndes, C. M., Unemo, M., & Members of the, N. C. S. C. (2013). Diagnostics, surveillance and management of sexually transmitted infections in Europe have to be improved: Lessons from the European Conference of National Strategies for Chlamydia trachomatis and Human papillomavirus (NSCP conference) in Latvia, 2011. Journal of the European Academy of Dermatology and Venereology, 27(10), 1308–1311. doi:10.1111/j.1468-3083.2012.04545.x.
Pan, Q., Pais, R., Ohandjo, A., He, C., He, Q., Omosun, Y., et al. (2015). Comparative evaluation of the protective efficacy of two formulations of a recombinant Chlamydia abortus subunit candidate vaccine in a mouse model. Vaccine, 33(15), 1865–1872. doi:10.1016/j.vaccine.2015.02.007.
Perez-Gil, J. (2008). Structure of pulmonary surfactant membranes and films: The role of proteins and lipid-protein interactions. Biochimica et Biophysica Acta, 1778(7–8), 1676–1695. doi:10.1016/j.bbamem.2008.05.003.
Picard, M. D., Cohane, K. P., Gierahn, T. M., Higgins, D. E., & Flechtner, J. B. (2012). High-throughput proteomic screening identifies Chlamydia trachomatis antigens that are capable of eliciting T cell and antibody responses that provide protection against vaginal challenge. Vaccine, 30(29), 4387–4393. doi:10.1016/j.vaccine.2012.01.017.
Porcella, S. F., Carlson, J. H., Sturdevant, D. E., Sturdevant, G. L., Kanakabandi, K., Virtaneva, K., et al. (2015). Transcriptional profiling of human epithelial cells infected with plasmid-bearing and plasmid-deficient Chlamydia trachomatis. Infection and Immunity, 83(2), 534–543. doi:10.1128/IAI.02764-14.
Qu, Y., Frazer, L. C., O’Connell, C. M., Tarantal, A. F., Andrews, C. W, Jr, O’Connor, S. L., et al. (2015). Comparable genital tract infection, pathology, and immunity in rhesus macaques inoculated with wild-type or plasmid-deficient Chlamydia trachomatis serovar D. Infection and Immunity,. doi:10.1128/IAI.00841-15.
Rank, R. G., Bowlin, A. K., Reed, R. L., & Darville, T. (2003). Characterization of chlamydial genital infection resulting from sexual transmission from male to female guinea pigs and determination of infectious dose. Infection and Immunity, 71(11), 6148–6154.
Rank, R. G., & Whittum-Hudson, J. A. (2010). Protective immunity to chlamydial genital infection: Evidence from animal studies. Journal of Infectious Diseases, 201(Suppl 2), S168–S177. doi:10.1086/652399.
Rey-Ladino, J., Ross, A. G., & Cripps, A. W. (2014). Immunity, immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis. Human Vaccines & Immunotherapeutics, 10(9), 2664–2673. doi:10.4161/hv.29683.
Saka, H. A., Thompson, J. W., Chen, Y. S., Dubois, L. G., Haas, J. T., Moseley, A., et al. (2015). Chlamydia trachomatis infection leads to defined alterations to the lipid droplet proteome in epithelial cells. PLoS ONE, 10(4), e0124630. doi:10.1371/journal.pone.0124630.
Sanadgol, N., Mostafaie, A., Mansouri, K., & Bahrami, G. (2012). Effect of palmitic acid and linoleic acid on expression of ICAM-1 and VCAM-1 in human bone marrow endothelial cells (HBMECs). Arch Med Sci, 8(2), 192–198. doi:10.5114/aoms.2012.28544.
Shimomura, H., Hosoda, K., Hayashi, S., Yokota, K., Oguma, K., & Hirai, Y. (2009). Steroids mediate resistance to the bactericidal effect of phosphatidylcholines against Helicobacter pylori. FEMS Microbiology Letters, 301(1), 84–94. doi:10.1111/j.1574-6968.2009.01807.x.
Sixt, B. S., Siegl, A., Muller, C., Watzka, M., Wultsch, A., Tziotis, D., et al. (2013). Metabolic features of Protochlamydia amoebophila elementary bodies—A link between activity and infectivity in Chlamydiae. PLoS Pathogens, 9(8), e1003553. doi:10.1371/journal.ppat.1003553.
Stubbs, C. D., & Smith, A. D. (1984). The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochimica et Biophysica Acta, 779(1), 89–137.
Subramaniam, S., Fahy, E., Gupta, S., Sud, M., Byrnes, R. W., Cotter, D., et al. (2011). Bioinformatics and systems biology of the lipidome. Chemical Reviews, 111(10), 6452–6490. doi:10.1021/cr200295k.
Szaszak, M., Chang, J. C., Leng, W., Rupp, J., Ojcius, D. M., & Kelley, A. M. (2013). Characterizing the intracellular distribution of metabolites in intact Chlamydia-infected cells by Raman and two-photon microscopy. Microbes and Infection, 15(6–7), 461–469. doi:10.1016/j.micinf.2013.03.005.
Torrone, E., Papp, J., Weinstock, H., & Centers for Disease, C., & Prevention. (2014). Prevalence of Chlamydia trachomatis genital infection among persons aged 14–39 years—United States, 2007–2012. Morbidity and Mortality Weekly Report, 63(38), 834–838.
Uhl, O., Demmelmair, H., Segura, M. T., Florido, J., Rueda, R., Campoy, C., et al. (2015). Effects of obesity and gestational diabetes mellitus on placental phospholipids. Diabetes Research and Clinical Practice, 109(2), 364–371. doi:10.1016/j.diabres.2015.05.032.
van Ooij, C., Kalman, L., van Ijzendoorn, S., Nishijima, M., Hanada, K., Mostov, K., et al. (2000). Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cellular Microbiology, 2(6), 627–637.
Vanrompay, D., Hoang, T. Q., De Vos, L., Verminnen, K., Harkinezhad, T., Chiers, K., et al. (2005). Specific-pathogen-free pigs as an animal model for studying Chlamydia trachomatis genital infection. Infection and Immunity, 73(12), 8317–8321. doi:10.1128/IAI.73.12.8317-8321.2005.
Vasilevsky, S., Greub, G., Nardelli-Haefliger, D., & Baud, D. (2014). Genital Chlamydia trachomatis: Understanding the roles of innate and adaptive immunity in vaccine research. Clinical Microbiology Reviews, 27(2), 346–370. doi:10.1128/CMR.00105-13.
Wali, S., Gupta, R., Veselenak, R. L., Li, Y., Yu, J. J., Murthy, A. K., et al. (2014). Use of a Guinea pig-specific transcriptome array for evaluation of protective immunity against genital chlamydial infection following intranasal vaccination in Guinea pigs. PLoS ONE, 9(12), e114261. doi:10.1371/journal.pone.0114261.
Wang, J., Zhang, Y., Lu, C., Lei, L., Yu, P., & Zhong, G. (2010). A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. Journal of Immunology, 185(3), 1670–1680. doi:10.4049/jimmunol.1001240.
Wylie, J. L., Hatch, G. M., & McClarty, G. (1997). Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. Journal of Bacteriology, 179(23), 7233–7242.
Yeruva, L., Myers, G. S., Spencer, N., Creasy, H. H., Adams, N. E., Maurelli, A. T., et al. (2014). Early microRNA expression profile as a prognostic biomarker for the development of pelvic inflammatory disease in a mouse model of chlamydial genital infection. MBio, 5(3), e01241-14. doi:10.1128/mBio.01241-14.
Zhou, B. R., Zhang, J. A., Zhang, Q., Permatasari, F., Xu, Y., Wu, D., et al. (2013). Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha via a NF-kappaB-dependent mechanism in HaCaT keratinocytes. Mediators of Inflammation, 2013, 530429. doi:10.1155/2013/530429.
Acknowledgments
We thank Dr. Roger Rank (Arkansas Children’s Hospital Research Institute) for sharing the 104C1 cell line, his support in establishing the guinea pig model and insightful discussions. We thank Drs. George R. Negrete and Oleg Larionov, Chemistry, UTSA for use of their equipment and reagents to prepare liposome encapsulated PC 16:0/18:1. This work was supported by National Institutes of Health (NIH) Grant (1RO3AI092621-01) and the Center for Excellence in Infection Genomics (CEIG) training Grant (DOD #W911NF-11-1-0136). Partial support of this study was from the Jane and Roland Blumberg Professorship in Biology for Dr. Arulanandam. Mass spectrometry analyses were conducted in the Metabolomics Core Facility of the Mass Spectrometry Laboratory at the University of Texas Health Science Center at San Antonio, with instrumentation funded in part by NIH Grant (1S10RR031586-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare in regards to this work.
Ethical approval
All Authors listed in this manuscript agree and comply with compliance with ethical requirements.
Additional information
Shradha Wali and Rishein Gupta have contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
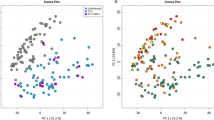
PCA of Chlamydia trachomatis infected guinea pigs. Clusters obtained by principal component analysis (PCA) of log-scaled polar metabolite data of genital swabs obtained from Ct D and mock infected guinea pigs at days (a) 3, (b) 9 and (c) 15 post challenge. Supplementary material 1 (TIFF 634 kb)
Table S1
Fold changes in representative species detected in various metabolite families following Chlamydia trachomatis D infection In vivo (swabs obtained from guinea pigs). Supplementary material 2 (DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Wali, S., Gupta, R., Yu, JJ. et al. Guinea pig genital tract lipidome reveals in vivo and in vitro regulation of phosphatidylcholine 16:0/18:1 and contribution to Chlamydia trachomatis serovar D infectivity. Metabolomics 12, 74 (2016). https://doi.org/10.1007/s11306-016-0998-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-0998-5