Abstract
Acute respiratory distress syndrome (ARDS) contributes substantially to mortality and morbidity in USA and worldwide. Due to limitations in early diagnostics of ARDS by classical methods, there has been need for discovery of novel methods and biomarkers for its characterization. We present here first high-resolution 1H nuclear magnetic resonance (NMR) metabolic profiling of serum from ARDS patients and non ARDS (NARDS) controls to search for novel biomarkers in blood serum for better diagnostics and prognostics. We have carried out study with serum samples from a total of 45 subjects, which included 26 ARDS patients and 19 NARDS controls. Principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA) were performed on 1H NMR spectra of serum for group discrimination between the two. PCA and PLS-DA on 1H NMR spectra of serum clearly discriminated between NARDS control and ARDS diseased samples. In our study, lipid resonances showed major contribution for this separation in loading plots. In order to highlight role of other small molecular weight metabolites, the analysis was also carried out after removing lipids resonances from NMR spectrum. There was significant increase in concentrations of N-acetylglycoproteins (NAC) (p = 0.001), acetoacetate (p = 0.001), lactate (p = 0.001), creatinine (p = 0.003), histidine (p = 0.03), formate (p = 0.04) and aromatic amino acids serum of ARDS patients. Along with small metabolites, lipids play a very important role in this discrimination and can differentiate between two classes. Our study has given new avenue in the metabolic profiling of lung injuries.
Similar content being viewed by others
1 Introduction
Acute respiratory distress syndrome (ARDS) is a perilous disease contributing significantly to mortality and morbidity in USA and worldwide. Incidence of acute lung injury (ALI) in the United States is reported to be 78.9/100,000 persons per year, with a mortality rate of approximately 40 % (Rubenfeld et al. 2005a). There are 190,600 new cases of ALI every year in U.S.A. alone (Rubenfeld et al. 2005b). Acute respiratory distress syndrome, first mentioned by Ashbaugh et al. (1967), is categorized in three different classes based on the P/F ratio (P- partial pressure of oxygen/F-fraction of inspired oxygen) and mortality rate. According to the recently accepted “Berlin definition”(The 2012), patients with P/F ratio between 200 and 300 and 27 % mortality belong to class of mild ARDS, P/F ratio between 100 and 200 with 32 % mortality to moderate ARDS and P/F ratio of less than 100 with 49 % mortality to severe ARDS. Berlin definition has eliminated the earlier class of ALI, ALI (P/F ratio between 200 and 300) and ARDS (P/F ratio less than 200) (Bernard et al. 1994). The etiology of ARDS is multi factorial (Petty 1982). The causative insults, mainly severe traumatic injury, severe head injury, injury to the chest, pneumonia and sepsis create lung injury through release of inflammatory mediators resulting in reduced oxygenation. Delay in diagnosis, thus treatment enhances the severity of ARDS (Jean-Ralph et al. 2000). Due to limitations in early diagnostics by classical methods, there have been demands for discovery of novel methods and biomarkers, which can be helpful in its characterization and facilitate interventions to prevent its progression to severe form. Researchers have been trying this from past four decades without substantial success (Bernard et al. 1994; Cribbs and Martin 2008; Donnelly et al. 1996; Geiser et al. 2001; Matthay et al. 2003; Tripathi et al. 2012).
Metabolomics is defined as qualitative and quantitative measurement of the multi parametric metabolic response of living systems to pathophysiological stimuli or genetic modification (Nicholson and Lindon 2008). Any change at genetic level will be reflected in cellular expression in form of mRNA, proteins and finally as last products as small molecular weight metabolites. In continuation of “OMICS” fields the study of total metabolites of cell has been christened as metabolomics by its discoverers. It is a constantly evolving field with new developments taking place regularly (MacKinnon et al. 2012; MacKinnon 2013; Somashekar et al. 2011). Metabolomics studies employ some key analytical techniques such as MS, GC-MS, LC-MS and NMR. Out of these NMR has been prominently used for discovery of small metabolites in body fluids (Nicholson et al. 1999). Metabolomics studies can provide a plethora of information about processes happening inside a particular organism at a time. A number of previous studies have elucidated role of metabolites present in body fluids and their association with various pathological conditions (Kaddurah-Daouk and Krishnan 2009; Kaddurah-Daouk et al. 2008; Nicholson and Wilson 2003). High-resolution 1H NMR metabolic profiling is a non-invasive, powerful technique having capability to identify a number of metabolites simultaneously with minimum sample processing required. This has played important role in significant studies for tissues and bio-fluids successfully. Different reports on cancer tissues (Whitehead and Kieber-Emmons 2005), liver abscess (Bharti et al. 2012), liver grafts (Tripathi et al. 2009), human urine (Gartland et al. 1990), follicular fluid (Pinero-Sagredo et al. 2010), bile (Ijare et al. 2005), plasma (Engan et al. 1990), and serum (Tzouvelekis et al. 2005) have given information about small molecular weight metabolites present in these tissues and fluids as well as discovery of novel biomarkers for particular pathological condition. Recently a study has utilized high-resolution 1H metabolic profiling in bronchoalveolar lavage fluid (BALF) for characterizing human lung injury (Rai et al. 2012). Only one effort have been made so far, for metabolic profiling of ARDS in serum using NMR, way back in 1989 Kuo et al. (1989). ARDS needed to be studied in the light of high-resolution 1H metabolic profiling. Here, we present first high-resolution 1H metabolic profiling of serum from the patients of ARDS in order to search for novel biomarkers for better diagnostics and prognostics. Our study has helped in identifying key metabolites such as valine, leucine and isoleucine, NAC, acetate, acetoacetate, glutamine, glutamate, lactate, creatinine, lysine, arginine, urea, uridine, formate, lipids and lipoproteins in serum responsible for separation of ARDS from healthy control.
2 Materials and methods
2.1 Human subjects
This study was conducted in intensive care unit (ICU) of a tertiary care medical centre and Centre of Biomedical Magnetic Resonance in northern India. Approval from institutes ethical committee and informed patient consent were taken before sample collection. All subjects diagnosed with ARDS at intensive care unit were enrolled in the study group. Data collection includes demographic profile, clinical characteristics and illness severity scores like Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment score (SOFA score) at admission. ARDS diagnostic criteria was based on Berlin definition (The 2012). Exclusion criteria in present study includes patients with age less than 18 years, pregnancy, chronic obstructive pulmonary disease (COPD) (McClay et al. 2010) patients, bronchial asthma, interstitial lung disease, and other chronic respiratory ailments.
NARDS controls (n = 19) were patients with healthy lungs, being ventilated for non-respiratory causes. We have not taken completely healthy controls because ventilator associated changes will dominate in the analysis. So, we have taken ventilated patients with healthy lungs as control. In control groups 12 out of 19 were patients with neuromuscular diseases, Guillain–Barré (GB) syndrome and 7 were suffering from pancreatitis. In all these control cases, lungs conditions were healthy prior to sample collection reflected by the P/F ratios averaging greater than 300. In ARDS group (n = 26) 7 patients were diagnosed with sepsis, 7 with pneumonia, 4 with malaria, 3 with chronic alcoholic and 5 with acute pancreatitis. Average P/F ratio for this diseased group was less than 207.
2.2 Sample collection
All serum samples were collected from blood samples of patients suffering from different stages of ARDS and from NARDS. Total 45 samples were collected which included 19 from NARDS controls and 26 from ARDS patients. Amount of blood collected was 2 ml for each sample. All samples were incubated for 30 min at room temperature and centrifuged (at 10 k rpm, 5 °C for 5 min). Golden yellow supernatant was collected and stored at −80 °C till NMR experiments.
2.2.1 NMR experimentation
All NMR spectra were recorded at Bruker Biospin Avance-III 800 MHz NMR (BrukerGmBH, Germany) spectrometer operating at proton frequency of 800.21 MHz equipped with CryoProbe. Serum samples were thawed just before acquiring NMR spectra and 450 μl of serum was taken in NMR tube. A co-axial insert containing 0.156 Mg in TSP solution (Sodium salt of trimethylsilyl-2,2,3,3-tetradeuteropropionicacid) was used for deuterium lock as well as for external standard reference.
2.2.2 One-dimensional NMR experiments
2.2.2.1 One-dimensional 1H spectra with Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence
One dimensional specta with CPMG (Carr, Purcell Carr and Purcell 1954; Meiboom and Gill 1958) sequence with water suppression were recorded to remove broad signals from lipids and proteins. CPMG spectra were recorded with 64 k time domain data points, 20.55 ppm spectral width, 128 scans, relaxation delay of 5 s, constant receiver gain value of 203 and 400 ms echo time. CPMG experiment provides spectra with smooth baseline, which facilitate multivariate chemo metric analysis. NMR spectra of all samples were recorded with constant parameters to ensure the accuracy of results. All NMR spectra were phased and baseline-corrected using Bruker Biospin TOPSPIN software (version 2.1).
2.2.2.2 One-dimensional spectra with water suppression
One-dimensional spectra with water suppression (zgpr in Bruker library) were recorded. This spectra contained broad humps (due to resonances from lipids and lipoproteins) in signal along with resonances from small molecular weight metabolites. All spectra were recorded with 64 k time domain data points, 20.55 ppm spectral width, 128 scans, relaxation delay of 5 s, and receiver gain value of 64. These spectra were used to get difference spectra (zgpr-CPMG) to get resonances from lipids and lipoproteins.
2.2.3 Two-dimensional NMR experiments
Two-dimensional homo-nuclear (1H-1H COSY and 1H-1H TOCSY) and hetero-nuclear (1H-13C HSQC) experiments were also performed to confirm resonance assignment. Following are the experimental parameters: normal COSY spectra were acquired with 2 k data points in t2 domain with spectral width of 10,683 Hz, 256 increments in t1 were collected with 16 transients, 2 s relaxation delay and water resonance was suppressed (Hanai 1991; Klomp et al. 2011) during the relaxation delay. Resulting data was zero-filled to 1 k points and ‘qsine’ window function was used in both dimensions. To record TOCSY, MLEV17 sequence was used for mixing. Other parameters for TOCSY: t2 dimension 2 k data points, with 10,683 Hz of spectral width and 256 t1 increments for 16 transients. 80 ms mixing time was used. Parameters for HSQC, 2 k data points were collected in t2 dimension over spectral width of 12019 Hz, 340 t1 increments were collected with 24 transients, relaxation delay of 2.0 s and with acquisition time of 80 ms. The resulting data were zero filled to 512 data points and were weighted with 90° shifted squared sine bell window functions in both dimensions prior to Fourier transformation.
2.2.4 Spectral assignment
1H NMR spectra of serum of both groups were assigned using previously reported literature values and data obtained from BMRB database (Biological Magnetic Resonance Data Bank http://www.bmrb.wisc.edu/). Various overlapping resonances in 1D spectrum were identified with the help of 2D experiments, which also elucidate the connectivity between different spin systems. Both homonuclear (TOCSY, COSY) and heteronuclear (HSQC) spectra were recorded and analyzed, SI Figure 1 and 2. Assignments of different resonances have been given with chemical shifts in SI Table 1.
2.3 Statistical analysis
A total of 46 samples (19 NARDS controls and 26 ARDS) were used for multivariate analysis. Principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA) analysis were performed by ‘The Unscrambler X’ software package (Version 10.0.1, Camo ASA, Norway). PCA was performed to transform a number of (possibly) correlated variables into a (smaller) number of uncorrelated variables called principal components and PLS-DA was performed in order to sharpen the separation between groups of observations. Samples were randomized for all the analysis performed. Binning of NMR data was performed using AMIX software (version 3.7.10, Bruker BioSpin, Switzerland) for the chemical shift regions of 0.3–4.5 ppm and 5.17–9 ppm. Variable size buckets were used to avoid small variations in chemical shifts. The region between 4.5 ppm and 5.17 ppm has been excluded from the study to avoid variability from water suppression. A total 851 continuous integral segments of equal width of 0.01 ppm were collected for further analysis. The data obtained was mean centered, scaled to total intensity. These buckets were integrated and normalized by adjusting the total area to unity to minimize the effect of variable sample dilution.
2.4 Quantitative analysis of metabolites responsible for class discrimination
Metabolites contributing for differences between NARDS control and diseased ARDS groups by multivariate statistical analysis were taken for quantitative analysis from CPMG spectra. For calculation of absolute concentrations from CPMG integral values previously reported method is used (Bharti et al. 2008). Resonances from metabolites were integrated with respect to TSP for each spectrum from NARDS control group and diseased groups. Standard error and p-values were calculated for each metabolite and plotted as shown in Table 1.
2.5 Sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE)
10 % SDS-PAGE was performed according to the method of Laemmli (1970) and SDS-PAGE picture is shown in Supporting Information Fig. 3.
3 Results and discussion
3.1 Metabolic profile of serum in ARDS patients
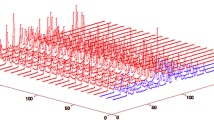
1H CPMG spectra of different groups have been shown in Fig. 1. NARDS control group has been represented with green and ARDS group with red color, respectively. These spectra were calculated from the mean of different spectra in each group. Resonances from various small metabolites with higher and lower intensities can be seen in both spectra. Amino acids such as leucine, isoleucine, valine, alanine, lysine, arginine, histidine etc. are present in both spectra. Aromatic amino acids peaks are more prominent in ARDS spectrum. ARDS spectrum shows many metabolites with higher peak intensity such as lipids, 2-methylbutyrate, acetate, acetoacetate, NAC (N-acetylglycoproteins), pyruvate, creatinine, urea/uridine, formate and aromatic amino acids. However alanine shows reduced intensity compared to control. Since visual interpretation of mean spectra of both groups may be deceptive, we performed multivariance analysis like PCA and PLS-DA, which explains role of variations in relative concentrations of various metabolites.
3.2 Multivariate analysis of 1H NMR spectra of serum; discrimination between NARDS control and diseased (ARDS) groups
We used 1H CPMG spectra for multivariate analysis of NARDS control and diseased (ARDS) groups for the role of small metabolites in separation. Difference spectrum (between 1D 1H spectrum and CPMG 1D 1H spectrum) was taken for multivariate analysis for elucidating the role of lipids and lipoproteins. First we performed PCA between NARDS control and diseased ARDS groups, Fig. 2a that shows separation between two groups in the PCA analysis. The first principal component (PC1) separated the sample and accounted for the 46 % of the variance within the data. The second principal component separated the data into control and ARDS groups and accounted for 22 % of the variance. The metabolites responsible for this separation of diseased and NARDS control groups have been represented in corresponding PC1 loading plot; Fig. 2b. Loading plot enables us to know how variables are associated with principal component. This loading plot shows dynamic change of metabolome within human blood serum of ARDS patients. All these metabolites were identified and labeled in loading plot. To sharpen the separation between these two groups, PLS-DA was performed which showed similar results as PCA with R2Y = 88 % and Q2 = 70 %, Fig. 2c. The loading plot depicting the role of small metabolites involved has been represented in Fig. 2d. The metabolites have been identified and labeled accordingly in the figure.
If we observe carefully PCA and PLS-DA loading plots, we observe that greater contribution comes from lipids (shown as lipid1 and lipid2). In order to access the contributions from low molecular weight metabolites, lipids resonances (0.8–0.92, 1.21–1.32, 1.96–2.00, 2.71–2.80 and 5.2–5.38 ppm) were removed from the bucket and multivariate analysis was carried out (Lanza et al. 2010; Rai et al. 2012). PCA analysis showed almost clear separation of NARDS control verses diseased groups, as shown in Supporting Information Fig. 4a. As we can see in PCA loading plot, branch chain amino acids (BCA) such as valine, leucine and isoleucine, NAC, acetate, acetoacetate, glutamine, glutamate, lactate, creatinine, lysine, arginine, urea, uridine, formate and aromatic amino acids have differences in concentration between two groups and separated diseased group patients from NARDS control as shown in Supporting Information Fig. 4b. Further, PLS-DA (R2Y = 85 % and Q2 = 69 %) was carried out for this data, which enhanced the separation between diseased and NARDS control groups. PLS-DA plot and corresponding loading plot has been shown in Supporting Information Fig. 4c and d.
Increase in serum C18 unsaturated free fatty acids has been reported earlier as a predictor of the development of ARDS (Bursten et al. 1996). Based upon above study, we analyzed our data in terms of contribution of lipid and lipoprotein content. We created difference spectra between 1H 1D and CPMG 1H 1D which provides resonances arising only due to macromolecules like lipids, low density lipoproteins (LDL), very low density lipoproteins (VLDL) which is shown in Supporting Information Fig. 5, (Sonkar et al. 2012).We performed multivariate analysis based primarily on lipid content. PCA analysis of this data showed better separation than earlier ones with first principal component (PC1) separating both groups with accounting 53 % variance within data, (Supporting Information Fig. 6a, b). PLS-DA (R2Y = 93 % and Q2 = 87 %) done for lipid spectrum also corroborated PCA results with NARDS control and diseased groups Supporting Information Fig. 6c and d. There was one outlier in PCA; this may be due to the reason that this particular patient underwent multiple surgeries.
From last 3–4 decades there has been immense need for new methods, which can be used for early diagnosis of ARDS. Recently (Rai et al. 2012) have showed 1H NMR profiling of bronchoalveolar lavage fluid (BALF) can be used as a good technique for identification of ALI from control group as well as differentiation of ALI and ARDS patients. In the present study we characterize ARDS based on serum metabolites. Metabolomics analysis of serum of ARDS diseased and NARDS control group provided us new insights about ARDS patients in terms of unique small metabolites present in their serum. Figure 2a and b give us qualitative information about molecules, which are varying in their intensities. Further quantification analysis gives actual picture of changes of concentrations of the molecules involved. Except alanine, other metabolites show enhanced concentration in ARDS, as shown in Fig. 3; Table 1. The change in concentration of these small molecules is the key factor for separation of ARDS from NARDS control group; Fig. 2 and Supporting Information Fig. 6. In rat animal system, alanine, acetate, acetoacetate, formate, lactate have been characterized to be biomarkers involved in sepsis, one of the leading causes of ARDS (Lin et al. 2009). Study in rats showed increase in concentrations of alanine, acetate, acetoacetate and lactate (Lin et al. 2009); in human ARDS serum we have found similar results except decrease in alanine concentration. In experimental rats, it has been demonstrated that serum alanine levels were reduced in the postpartum period following lung injury and external alanine administration was suggested for proper healing (Karadayi et al. 2012). Increase in concentration of lactate in ARDS has been reported earlier in case of lung injury (De Backer et al. 1997). BCA are product of protein breakdown which happens due to injury and infection of lungs (Garlick and Grant 1988). This is anticipated in case of lung injury and our results are consistent with previous studies (Freund et al. 1978). Similarly increase in concentration of other amino acids may be due to protein breakdown.
We have calculated and plotted concentrations of key metabolites in NARDS and ARDS serum, which have been represented in terms of micromoles/liter or au (concentration with respect to TSP) (Fig. 3; Table 1). Concentrations of small metabolites present in serum of NARDS control are in similar range as reported earlier (Psychogios et al. 2011). Concentrations of lipids (from region 1 and 2), acetate, creatinine and histidine have become almost double in case of ARDS serum. Branch chain amino acids show slight increase while concentration of histidine and formate increase drastically in ARDS serum in comparison to serum of NARDS control (Fig. 3; Table 1). Acetoacetate concentration is also significantly enhanced in ARDS patients. This a type of ketone body which may be generated due to β-oxydation of fatty acids to meet the energy demand (Lin et al. 2009). Increase in creatinine concentration is associated with muscle damage (Wyss and Kaddurah-Daouk 2000). Increase in serum C-18 unsaturated free fatty acids have been proved to be an indicator of development of ARDS (Bursten et al. 1996). Serum lipopolysachharide binding protein level has also been correlated with severity and mortality of patients with severe sepsis (Opal et al. 1999; Villar et al. 2009). Sodium dodecyl sulfate (SDS) gel picture also shows variation in number and intensities of different proteins in serum of healthy control, NARDS control and ARDS (Supporting Information Fig. 3). The results are encouraging and provide us an independent biomarker without need of any separation and purification.
4 Conclusion
Based on the above results and followed discussion, we conclude that 1H profiling of serum is potentially important diagnostic tool. We have shown change in serum metabolome of ARDS patients can be utilized to discriminate them from respective control samples. Key metabolites involved have been identified and their role in ARDS has been discussed. Lipid and lipoprotein content can be taken as independent biomarkers for assessment of ARDS in lung injury patients. This is the first study of this kind and will lead to better diagnostic and management method of ARDS. This pilot study will encourage various types of studies in ARDS i.e. infection associated changes and related small metabolites.
5 Supporting Information
Various 2D NMR spectrum confirming the assignments, Table of assigned metabolites and table showing patient characteristic data sheet are given in supporting information.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- NMR:
-
Nuclear magnetic resonance
- NARDS:
-
Non ARDS
- PCA:
-
Principal component analysis
- PLS-DA:
-
Partial least square discriminant analysis
- NAC:
-
N-Acetylglycoproteins
- ALI:
-
Acute lung injury
- P/F:
-
(P-partial pressure of oxygen/F-fraction of inspired oxygen)
- ICU:
-
Intensive care unit
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- SOFA:
-
Sequential Organ Failure Assessment
- COPD:
-
Chronic obstructive pulmonary disease
- GB:
-
Guillain–Barré
- CPMG:
-
Carr–Purcell–Meiboom–Gill
- SDS-PAGE:
-
Sodium dodecylsulphate polyacrylamide gel electrophoresis
- LDL:
-
Low density lipoproteins
- VLDL:
-
Very low density lipoproteins
- PC1:
-
Principal component 1
- BALF:
-
Bronchoalveolar lavage fluid
- au:
-
Arbitrary unit
References
Ashbaugh, D. G., Bigelow, D. B., Petty, T. L., & Levine, B. E. (1967). Acute respiratory distress in adults. Lancet, 2, 319–323.
Bernard, G. R., et al. (1994). Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive care medicine, 20, 225–232.
Bharti, S., Sinha, N., Joshi, B., Mandal, S., Roy, R., & Khetrapal, C. (2008). Improved quantification from 1H-NMR spectra using reduced repetition times. Metabolomics, 4, 367–376. doi:10.1007/s11306-008-0130-6.
Bharti, S., et al. (2012). Metabolomic profiling of amoebic and pyogenic liver abscesses: an in vitro NMR study. Metabolomics, 8, 540–555. doi:10.1007/s11306-011-0339-7.
Bursten, S. L., et al. (1996). An increase in serum C18 unsaturated free fatty acids as a predictor of the development of acute respiratory distress syndrome. Critical Care Medicine, 24, 1129–1136.
Carr, H. Y., & Purcell, E. M. (1954). Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Physical Review, 94, 630–638.
Cribbs, S. K., & Martin, G. S. (2008). Biomarkers in acute lung injury: are we making progress? Critical Care Medicine, 36, 2457–2459. doi:10.1097/CCM.0b013e318181052a.
De Backer, D., Creteur, J., Zhang, H., Norrenberg, M., & Vincent, J. L. (1997). Lactate production by the lungs in acute lung injury. American journal of respiratory and critical care medicine, 156, 1099–1104.
Donnelly, S. C., et al. (1996). The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Annals of internal medicine, 125, 191–196.
Engan, T., Krane, J., Klepp, O., & Kvinnsland, S. (1990). Proton nuclear magnetic resonance spectroscopy of plasma from healthy subjects and patients with cancer. New England Journal of Medicine, 322, 949–953. doi:10.1056/NEJM199004053221402.
Freund, H. R., Ryan, J. A, Jr, & Fischer, J. E. (1978). Amino acid derangements in patients with sepsis: treatment with branched chain amino acid rich infusions. Annals of surgery, 188, 423–430.
Garlick, P. J., & Grant, I. (1988). Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochemical Journal, 254, 579–584.
Gartland, K. P., Sanins, S. M., Nicholson, J. K., Sweatman, B. C., Beddell, C. R., & Lindon, J. C. (1990). Pattern recognition analysis of high resolution 1H NMR spectra of urine. A nonlinear mapping approach to the classification of toxicological data. NMR in Biomedicine, 3, 166–172.
Geiser, T., Atabai, K., Jarreau, P. H., Ware, L. B., Pugin, J., & Matthay, M. A. (2001). Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. American Journal of Respiratory and Critical Care Medicine, 163, 1384–1388.
Hanai, T. (1991). Liquid chromatography in biomedical analysis. Kyoto: Elsevier.
Ijare, O. B., Somashekar, B. S., Gowda, G. A., Sharma, A., Kapoor, V. K., & Khetrapal, C. L. (2005). Quantification of glycine and taurine conjugated bile acids in human bile using 1H NMR spectroscopy. Magnetic resonance in medicine, 53, 1441–1446. doi:10.1002/mrm.20513.
Jean-Ralph, Z., et al. (2000). Delayed treatment contributes to mortality in ICU patients with severe active pulmonary tuberculosis and acute respiratory failure. Intensive Care Medicine, 27, 513–520. doi:10.1007/s001340000849.
Kaddurah-Daouk, R., & Krishnan, K. R. (2009). Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology, 34, 173–186. doi:10.1038/npp.2008.174.
Kaddurah-Daouk, R., Kristal, B. S., & Weinshilboum, R. M. (2008). Metabolomics: A global biochemical approach to drug response and disease. Annual Review of Pharmacology and Toxicology, 48, 653–683. doi:10.1146/annurev.pharmtox.48.113006.094715.
Karadayi, S., et al. (2012). Investigation of wound healing in rat lung tissues in the postpartum period. Experimental and Therapeutic Medicine, 3, 807–810.
Klomp, D. W. J., et al. (2011). 31P MRSI and 1H MRS at 7 T: Initial results in human breast cancer. NMR in Biomedicine, 24, 1337–1342.
Kuo, C. D., Wu, W. G., Wang, J. H., Chen, S. M., & Chiang, B. N. (1989). Proton nuclear magnetic resonance studies of plasma to determine metabolic status of patients with adult respiratory distress syndrome. Clinical Chemistry, 35, 667–670.
Laemmli, U. K. (1970). Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature, 227, 680–685.
Lanza, I. R., Zhang, S., Ward, L. E., Karakelides, H., Raftery, D., & Nair, K. S. (2010). Quantitative Metabolomics by <sup>1</sup>H-NMR and LC-MS/MS Confirms Altered Metabolic Pathways in Diabetes. PLoS ONE, 5, e10538. doi:10.1371/journal.pone.0010538.
Lin, Z.-Y., et al. (2009). A metabonomic approach to early prognostic evaluation of experimental sepsis by 1H NMR and pattern recognition. NMR in Biomedicine, 22, 601–608. doi:10.1002/nbm.1373.
MacKinnon, N., Khan, A., Chinnaiyan, A., Rajendiran, T., & Ramamoorthy, A. (2012). Androgen receptor activation results in metabolite signatures of an aggressive prostate cancer phenotype: An NMR-based metabonomics study. Metabolomics, 8, 1026–1036. doi:10.1007/s11306-012-0398-4.
MacKinnon, N., et al. (2013). MetaboID: A graphical user interface package for assignment of 1H NMR spectra of bodyfluids and tissues. Journal of Magnetic Resonance, 226, 93–99. doi:10.1016/j.jmr.2012.11.008.
Matthay, M. A., et al. (2003). Future Research Directions in Acute Lung Injury: Summary of a National Heart, Lung, and Blood Institute Working Group. American Journal of Respiratory and Critical Care Medicine, 167, 1027–1035. doi:10.1164/rccm.200208-966WS.
McClay, J. L., et al. (2010). (1)H nuclear magnetic resonance metabolomics analysis identifies novel urinary biomarkers for lung function. Journal of Proteomics, 9, 3083–3090. doi:10.1021/pr1000048.
Meiboom, S., & Gill, D. (1958). Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Review of Scientific Instruments, 29, 688–691.
Nicholson, J. K., & Lindon, J. C. (2008). Systems biology: Metabonomics. Nature, 455, 1054–1056.
Nicholson, J. K., Lindon, J. C., & Holmes, E. (1999). ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica, 29, 1181–1189. doi:10.1080/004982599238047.
Nicholson, J. K., & Wilson, I. D. (2003). Opinion: understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nature Reviews Drug Discovery, 2, 668–676. doi:10.1038/nrd1157nrd1157.
Opal, S. M., et al. (1999). Relationship between Plasma Levels of Lipopolysaccharide (LPS) and LPS-Binding Protein in Patients with Severe Sepsis and Septic Shock. Journal of Infectious Diseases, 180, 1584–1589. doi:10.1086/315093.
Petty, T. L. (1982). Adult respiratory distress syndrome: definition and historical perspective. Clinics in Chest Medicine, 3, 3–7.
Pinero-Sagredo, E., Nunes, S., de Los Santos, M. J., Celda, B., & Esteve, V. (2010). NMR metabolic profile of human follicular fluid. NMR Biomed, 23, 485–495. doi:10.1002/nbm.1488.
Psychogios, N., et al. (2011). The Human Serum Metabolome. PLoS ONE, 6, e16957. doi:10.1371/journal.pone.0016957.
Rai, R., et al. (2012). Metabolic profiling in human lung injuries by high-resolution nuclear magnetic resonance spectroscopy of bronchoalveolar lavage fluid (BALF). Metabolomics, 1–10, 667–676. doi:10.1007/s11306-012-0472-y.
Rubenfeld, G. D., et al. (2005a). Incidence and Outcomes of Acute Lung Injury. New England Journal of Medicine, 353, 1685–1693. doi:10.1056/NEJMoa050333.
Rubenfeld, G. D., et al. (2005b). Incidence and Outcomes of Acute Lung Injury. New England Journal of Medicine, 353, 1685–1693. doi:10.1056/NEJMoa050333.
Somashekar, B. S., et al. (2011). Magic Angle Spinning NMR-Based Metabolic Profiling of Head and Neck Squamous Cell Carcinoma Tissues. Journal of Proteome Research, 10, 5232–5241. doi:10.1021/pr200800w.
Sonkar, K., Behari, A., Kapoor, V. K., & Sinha, N. (2012). 1H NMR metabolic profiling of human serum associated with benign and malignant gallstone diseases. Metabolomics, 1–14, 515–528. doi:10.1007/s11306-012-0468-7.
The, A. D. T. F. (2012). Acute respiratory distress syndrome: The berlin definition. JAMA: The. Journal of the American Medical Association, 307, 2526–2533. doi:10.1001/jama.2012.5669.
Tripathi, P., Bala, L., Saxena, R., Yachha, S. K., Roy, R., & Khetrapal, C. L. (2009). 1H NMR spectroscopic study of blood serum for the assessment of liver function in liver transplant patients. Journal of gastrointestinal and liver diseases, 18, 329–336.
Tripathi, P., et al. (2012). Delineating metabolic signatures of head and neck squamous cell carcinoma: Phospholipase A2, a potential therapeutic target. The International Journal of Biochemistry & Cell Biology, 44, 1852–1861. doi:10.1016/j.biocel.2012.06.025.
Tzouvelekis, A., Kouliatsis, G., Anevlavis, S., & Bouros, D. (2005). Serum biomarkers in interstitial lung diseases. Respiratory Research, 6, 78. doi:10.1186/1465-9921-6-78.
Villar, J., et al. (2009). Serum Lipopolysaccharide Binding Protein Levels Predict Severity of Lung Injury and Mortality in Patients with Severe Sepsis. PLoS ONE, 4, e6818. doi:10.1371/journal.pone.0006818.
Whitehead, T. L., & Kieber-Emmons, T. (2005). Applying in vitro NMR spectroscopy and 1H NMR metabonomics to breast cancer characterization and detection. Progress in Nuclear Magnetic Resonance Spectroscopy, 47, 165–174.
Wyss, M., & Kaddurah-Daouk, R. (2000). Creatine and Creatinine Metabolism. Physiological Reviews, 80, 1107–1213.
Acknowledgments
Chandan Singh and Ratan Kumar Rai acknowledge financial support from Council of Scientific and Industrial Research, India. We also acknowledge our statistician Dr. S.K. Mandal for his support in statistical analysis. This work was financially support from Department of Biotechnology, India (Grant No. BT/PR12700/BRB/10/719/2009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, C., Rai, R.K., Azim, A. et al. Metabolic profiling of human lung injury by 1H high-resolution nuclear magnetic resonance spectroscopy of blood serum. Metabolomics 11, 166–174 (2015). https://doi.org/10.1007/s11306-014-0688-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-014-0688-0