Abstract
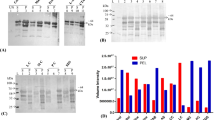
Interferon-γ (IFN-γ) is a broad-spectrum antiviral glycoprotein that produced by lymphatic T cells and natural killer cells those who had stimulated by antigen. Human IFN-γ (hIFN-γ) often used in clinical research and practice because of its bioactivity, for example, antivirus, antitumor, controlling cell apoptosis, and the strict selectivity. However, due to the difficulties of Escherichia coli expression system meet in protein folding, the hIFN-γ often existed as inclusion body. The production of soluble hIFN-γ can be developed to shorten the production cycle and decrease the cost. In this study, small ubiquitin-related modifier fusion technology was used to express and purify recombinant hIFN-γ. Expression induced by adding 50 mM arginine and 1 % (w/v) glycerol into the culture at 24 °C existed as a soluble form of 70 % in total protein. Finally, about 62 mg recombinant hIFN-γ was obtained from 1 L fermentation culture with no less than 96 % purity. Determined by cytopathic effect inhibition assay, the specific activity of the recombinant hIFN-γ achieved at 7.78 × 105 IU/mL.








Similar content being viewed by others
References
Arora D, Khanna N (1996) Method for increasing the yield of properly folded recombinant human gamma interferon from inclusion bodies. J Biotechnol 52:127–133
Blankenstein T, Qin ZH (2003) The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol 15:148–154. doi:10.1016/s0952-7915(03)00007-4
Butt TR, Edavettal SC, Hall JP, Mattern MR (2005) SUMO fusion technology for difficult-to-express proteins. Protein Expr Purif 43:1–9
Gadgil M, Kapur V, Hu WS (2005) Transcriptional response of Escherichia coli to temperature shift. Biotechnol Prog 21:689–699
Ghosh S, Rasheedi S, Rahim SS (2004) Method for enhancing solubility of the expressed recombinant proteins in Escherichia coli. Biotechniques 37(3):418–424
Gough DJ, Levy DE, Johnstone RW, Clarke CJ (2008) IFN-gamma signaling—does it mean JAK-STAT? Cytokine Growth Factor Rev 19:383–394. doi:10.1016/j.cytogfr.2008.08.004
Lee CD, Sun HC, Hu SM, Chiu CF, Homhuan A, Liang SM, Leng CH, Wang TF (2008) An improved SUMO fusion protein system for effective production of native proteins. Protein Sci 17:1241–1248
Li JF, Zhang J, Zhang Z, Ma HW, Zhang JX, Zhang SQ (2010) Production of bioactive human beta-defensin-4 in Escherichia coli using SUMO fusion partner. Protein J 29:314–319. doi:10.1007/s10930-010-9254-4
Peroutka IRJ, Orcutt SJ, Strickler JE, Butt TR (2011) SUMO fusion technology for enhanced protein expression and purification in prokaryotes and eukaryotes. Methods Mol Biol 705:15
Rubinstein S, Familletti PC, Pestka S (1981) Convenient assay for interferons. J Virol 37:755
Shtrichman R, Samuel CE (2001) The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol 4:251–259. doi:10.1016/s1369-5274(00)00199-5
Wu Y, Fan Y, Xue B, Luo L, Shen J, Zhang S, Jiang Y, Yin Z (2006) Human glutathione S-transferase P1–1 interacts with TRAF2 and regulates TRAF2-CASK1 signals. Oncogene 25:5787–5800
Yoon SO, Hirata RDC, da Silva ACR, Nguyen NY, Hirata MH (2002) Cloning and expression of soluble recombinant protein comprising the extracellular domain of the human type I interferon receptor 2c subunit (IFNAR-2c) in E. coli. Biotechnol Lett 24:1443–1448
Acknowledgments
This work was financially supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD No. 164320H106).
Author information
Authors and Affiliations
Corresponding author
Additional information
Fenfen Zhu and Qi Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, F., Wang, Q., Pu, H. et al. Optimization of soluble human interferon-γ production in Escherichia coli using SUMO fusion partner. World J Microbiol Biotechnol 29, 319–325 (2013). https://doi.org/10.1007/s11274-012-1185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1185-0