Abstract
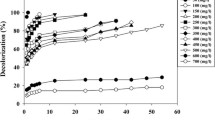
The objectives of this study were to investigate: (1) the capacity of Enterococcus faecalis on the decolorization of the azo dyes Acid Red 27 and Reactive Red 2; and (2) the growth characteristics of E. faecalis on those dyes. E. faecalis was able to decolorize Acid Red 27 and Reactive Red 2 effectively. High decolorization efficiency (95–100%) was achieved within 3 h of incubation for Acid Red 27, and 12 h for Reactive Red 2, at room temperature, neutral pH, static and non-aerated condition. Growth characteristics of E. faecalis on azo dyes, which were indicated by cell growth rate, biomass production, and growth yield, was worse than the control. E. faecalis grew better on Acid Red 27 rather than Reactive Red 2.





Similar content being viewed by others
References
Chang J-S, Chien C, Lin Y-C, Lin P-J, Ho J-Y (2001) Kinetic characteristics of bacterial azo dye decolorization by Pseudomonas luteola. Water Res 35:2841–2850
Chen H, Wang R-F, Cerniglia CE (2004) Molecular cloning, overexpression, purification, and characterization of an aerobic FMN-dependent azoreductase from Enterococcus faecalis. Protein Expr Purif 34:302–310
Coughlin MF, Kinkle BK, Bishop PL (1999) Degradation of azo dyes containing aminonaphthol by Sphingomonas sp. strain 1CX. J Indus Microbiol Biotechnol 23:341–346
Dos Santos AB (2004) Reductive decolourisation of dyes by thermophilic anaerobic granular sludge. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands
He F, Hu W, Li Y (2004) Biodegradation mechanisms and kinetic of azo dyes 4BS by a microbial consortium. Chemosphere 57:293–301
Kapdan IK, Kargi F, MacMullan G, Marchant R (2000) Effects of environmental conditions on biological decolorization of textile dyestuff by C. versicolor. Enzyme Microbial Technol 26:381–387
Keck A, Klein J, Kudlich M, Stolz A, Knackmuss H-J, Mattes R (1997) Reduction of azo dyes by redox mediators originating in the naphthalenesulfonic acid degradation pathway of Sphingomonas sp. strain BN6. Appl Environ Microbiol 63:3684–3690
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2005) Decolorization of various azo dyes by bacterial consortium. Dyes Pigments 67:55–61
Kim S-J, Ishikawa K, Hirai M, Shoda M (1995) Characterictics of a newly isolated fungus Geotrichum candidum Dec 1 which decolorizes various dyes. J Ferment Bioeng 79:601–607
Kodam KM, Soojhawon I, Lokhande PD, Gawai KR (2005) Microbial decolorization of reactive azo dyes under aerobic conditions. World J Microbiol Biotechnol 21:367–370
López C, Valade AG, Combourieu B, Mielgo I, Bouchon B, Lema JM (2004) Mechanism of enzymatic degradation of the azo dye Orange II determined by ex situ 1H Nuclear Magnetic Resonance and Electrospray Ionization-Ion Trap Mass Spectrometry. Anal Biochem 335:135–149
Mèndez-Paz D, Omil F, Lema JM (2005) Anaerobic treatment of azo dye Acid Orange 7 under batch condition. Enzyme Microb Technol 36:264–272
Miao Y (2005) Biological remediation of dyes in textile effluent: a review on current treatment technologies. http://www.ccee.iastate.edu/courses/ce521/yongjie.pdf
Novotny Č, Rawal B, Bhatt M, Patel M, Šašek V, Molitoris HP (2001) Capacity of Irpex lacteus and Pleurotus ostreatus for decolorization of chemically different dyes. J Biotechnol 89:113–122
Rafii F, Franklin W, Cerniglia C (1990) Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol 56:2146–2151
Ramalho PA, Cardoso MH, Cavaco-Paulo A, Ramalho MT (2004) Characterization of azo reduction activity in a novel Ascomycete yeast strain. Appl Environ Microbiol 70:2279–2288
Sani RK, Banerjee UC (1999) Decolorization of triphenylmethane dyes and textile and dyestuff effluent by Kurthia sp. Enzyme Microb Technol 24:433–437
Sponza DT, Işik M (2004) Decolorization and inhibition kinetic of Direct Black 38 azo dye with granulated anaerobic sludge. Enzyme Microb Technol 34:147–158
Sumathi S, Manju BS (2000) Uptake of reactive textile dyes by Aspergillus foetidus. Enzyme Microb Technol 27:347–355
Supaka N, Juntongjin K, Damronglerd S, Delia ML, Strehaiano P (2004) Microbial decolorization of reactive azo dyes in a sequential anaerobic-aerobic system. Chem Eng J 99:169–176
Sweeney EA, Chipman JK, Forsythe SJ (1994) Evidence for direct-acting oxidative genotoxicity by reduction products of azo dyes. Environ Health Perspect 102:119–122
Tan NCG (2001) Integrated and sequential anaerobic/aerobic biodegradation of azo dyes. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands
Van der Zee FP (2002) Anaerobic azo dye reduction. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands
Acknowledgement
The authors are grateful to acknowledge Yoga Aji Handoko and Suryasatrya Trihandaru for their technical helps, and Aldi Lasso and Chris Lundry for manuscript correction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Handayani, W., Meitiniarti, V.I. & Timotius, K.H. Decolorization of Acid Red 27 and Reactive Red 2 by Enterococcus faecalis under a batch system. World J Microbiol Biotechnol 23, 1239–1244 (2007). https://doi.org/10.1007/s11274-007-9355-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9355-1