Abstract
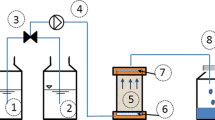
Granular drainage filters may provide new innovative solutions for removing phosphorus (P) from agricultural tile drainage water. The influence of filter sorbent properties is well recognized; however, intragranular diffusion may be critical for the P retention efficiency in drainage filters, where convective velocities are generally high. This was investigated in granular materials (Leca, Filtralite-P®, granulated Limestone, crushed Seashells, calcined diatomite earth (CDE), and poorly ordered Fe hydroxide aggregates (CFH)) with known solute transport behavior at variable flow rates. Phosphorus retention and release were investigated during continuous P loading with a constant inlet concentration of 0.3 mg P L−1 followed by a subsequent P release phase. A parallel batch sorption experiment ranked the materials according to strength of P retention CFH > Filtralite-P® > Seashells > Limestone > Leca, while CDE was a P source at low solution concentrations. Column experiments demonstrated that filters with non-equilibrium transport (CFH and Seashells) attained higher P retention rates and lower P release rates compared to filters with equilibrium flow (Filtralite-P® and Limestone). This behavior was even more pronounced as flow rate increased and was attributed to intragranular diffusion of P in CFH and Seashells, which would maintain concentration gradients between mobile convective water and immobile domains. This facilitated further diffusion and thus increased P retention. In addition, the release of previously retained P was limited in filters with non-equilibrium transport (<2 %) compared to the other filters (>5 %). The results demonstrated that intragranular diffusion was an important mechanism increasing P retention.



Similar content being viewed by others
Abbreviations
- P:
-
Phosphorus
- CFH:
-
Ferric hydroxide granules
- CDE:
-
Calcined diatomite earth
- ADW:
-
Artificial agricultural drainage water
References
Adam, K., Sovik, A. K., & Krogstad, T. (2006). Sorption of phosphorous to Filtralite-P (TM) - The effect of different scales. Water Resources, 40(6), 1143–1154. doi:10.1016/j.watres.2006.01.009.
Andersen, H. E., Larsen, S. E., Kronvang, B., Hansen, K. M., Laubel, A., Windolf, J., & Muus, K. (2006). Fosfat i drænvand. Vand og Jord, 13(4), 152–156 (In Danish).
Atkinson, A. C. (1994). Transforming both sides of a tree. The American Statistician, 48(4), 307–313. doi:10.2307/2684841.
Axe, L., & Trivedi, P. (2002). Intraparticle surface diffusion of metal contaminants and their attenuation in microporous amorphous Al, Fe, and Mn oxides. Journal of Colloid and Interface Science, 247(2), 259–265. doi:10.1006/jcis.2001.8125.
Barrow, N. J. (1983). A mechanistic model for describing the sorption and desorption of phosphate by soil. Journal of Soil Science, 34(4), 733–750.
Barrow, N. J. (2008). The description of sorption curves. European Journal of Soil Science, 59(5), 900–910. doi:10.1111/j.1365-2389.2008.01041.x.
Bolan, N. S., Barrow, N. J., & Posner, A. M. (1985). Describing the effect of time on sorption of phosphate by iron and aluminium hydroxides. Journal of Soil Science, 36(2), 187–197. doi:10.1111/j.1365-2389.1985.tb00323.x.
Brusseau, M. L. (1994). Transport of reactive contaminants in heterogeneous porous media. Reviews of Geophysics, 32(3), 285–313.
Canga, E., Iversen, B. V., & Kjaergaard, C. (2014). A simplified transfer function for estimating saturated hydraulic conductivity of porous drainage filters. Water, Air, and Soil Pollution, 225(1), 1–13. doi:10.1005/s11270-013-1794-8.
Canga, E., Kjaergaard, C., Iversen B.V., & Heckrath, G.J. (2016). Agricultural drainage filters. I. Filter hydro-physical properties and tracer transport. Water Air and Soil Pollution, (in review).
Chardon, W. J., Groenenberg, J. E., Temminghoff, E. J. M., & Koopmans, G. F. (2012). Use of reactive materials to bind phosphorus. Journal of Environmental Quality, 41(3), 636–646. doi:10.2134/jeq2011.0055.
Claveau-Mallet, D., Wallace, S., & Comeau, Y. (2013). Removal of phosphorus, fluoride and metals from a gypsum mining leachate using steel slag filters. Water Resources, 47(4), 1512–1520. doi:10.1016/j.watres.2012.11.048.
Diaz, J., Ingall, E., Benitez-Nelson, C., Paterson, D., de Jonge, M. D., McNulty, I., et al. (2008). Marine polyphosphate: A key player in geologic phosphorus sequestration. Science, 320(5876), 652–655. doi:10.1126/science.1151751.
Dzombak, D. A., & Morel, F. M. M. (1990). Surface complexation modeling: hydrous ferric oxide. New York: John Wiley & Sons.
Eastman, M., Gollamudi, A., Stämpfli, N., Madramootoo, C. A., & Sarangi, A. (2010). Comparative evaluation of phosphorus losses from subsurface and naturally drained agricultural fields in the Pike River watershed of Quebec, Canada. Agricultural Water Management, 97(5), 596–604. doi:10.1016/j.agwat.2009.11.010.
Egemose, S., Sonderup, M. J., Beinthin, M. V., Reitzel, K., Hoffmann, C. C., & Flindt, M. R. (2012). Crushed concrete as a phosphate binding material: a potential new management tool. Journal of Environmental Quality, 41(3), 647–653. doi:10.2134/jeq2011.0134.
Eveborn, D., Gustafsson, J. P., Hesterberg, D., & Hillier, S. (2009). XANES Speciation of P in environmental samples: An assessment of filter media for on-site wastewater treatment. Environmental Science and Technology, 43(17), 6515–6521. doi:10.1021/es901084z.
Fesch, C., Simon, W., Haderlein, S. B., Reichert, P., & Schwarzenbach, R. P. (1998). Nonlinear sorption and nonequilibrium solute transport in aggregated porous media: Experiments, process identification and modeling. Journal of Contaminant Hydrology, 31(3), 373–407.
Ford, R. G., Scheinost, A. C., & Sparks, D. L. (2001). Frontiers in metal sorption/precipitation mechanisms on soil mineral surfaces. Advances in Agronomy, 74, 41–62.
Goldberg, S., & Sposito, G. (1984). A chemical model of phosphate adsorption by soils: I. Reference oxide minerals. Soil Science Society of America Journal, 48(4), 772–778.
Groenenberg, J. E., Chardon, W. J., & Koopmans, G. F. (2013). Reducing phosphorus loading of surface water using iron-coated sand. Journal of Environmental Quality, 42(1), 250–259. doi:10.2134/jeq2012.0344.
Hay, M. B., Stoliker, D. L., Davis, J. A., & Zachara, J. M. (2011). Characterization of the intragranular water regime within subsurface sediments: Pore volume, surface area, and mass transfer limitations. Water Resources Research, 47(10). doi:10.1029/2010WR010303
Heathwaite, A. L., & Dils, R. M. (2000). Characterising phosphorus loss in surface and subsurface hydrological pathways. Science of the Total Environment, 251, 523–538. doi:10.1016/S0048-9697(00)00393-4.
Heckrath, G., Brookes, P. C., Poulton, P. R., & Goulding, K. W. T. (1995). Phosphorus leaching from soils containing different phosphorus concentrations in the Broadbalk experiment. Journal of Environmental Quality, 24(5), 904–910. doi:10.2134/jeq1995.00472425002400050018x.
Jenssen, P. D., Krogstad, T., Paruch, A. M., Mæhlum, T., Adam, K., Arias, C., et al. (2010). Filter bed systems treating domestic wastewater in the Nordic countries - Performance and reuse of filter media. Ecological Engineering, 36(12), 1651–1659. doi:10.1016/j.ecoleng.2010.07.004.
Johansson, L. (1997). The use of LECA (light expanded clay aggregrates) for the removal of phosphorus from wastewater. Water Science and Technology, 35(5), 87–93. doi:10.1016/S0273-1223(97)00056-5.
Kafkafi, U. (1972). Soil phosphorus. Analytical chemistry of phosphorus compounds (pp. 727–741). New York: Wiley & Sons.
Kettl, S. (1991). Accounting for heteroscedasticity in the transform both sides regression model. Applied Statistics, 40(2), 261–268. doi:10.2307/2347591.
Kirkkala, T., Ventelä, A. M., & Tarvainen, M. (2012a). Fosfilt filters in an agricultural catchment: a long-term field-scale. Agricultural and Food Science, 21(3), 237–246.
Kirkkala, T., Ventelä, A. M., & Tarvainen, M. (2012b). Long-term field-scale experiment on using lime filters in an agricultural catchment. Journal of Environmental Quality, 41(2), 410–419. doi:10.2134/jeq2010.0429.
Kleinman, P. J., Sharpley, A. N., McDowell, R. W., Flaten, D. N., Buda, A. R., & Tao, L. (2011). Managing agricultural phosphorus for water quality protection: principles for progress. Plant and Soil, 349, 169–182. doi:10.1007/s11104-011-0832-9.
Kleinman, P. J., Sharpley, A. N., Veith, T. L., Maguire, R. O., & Vadas, P. A. (2004). Evaluation of phosphorus transport in surface runoff from packed soil boxes. Journal of Environmental Quality, 33(4), 1413–1423.
Klimeski, A., Chardon, W. J., Turtola, E., & Uusitalo, R. (2012). Potential and limitations of phosphate retention media in water protection: A process-based review of laboratory and field-scale tests. Agricultural and Food Science, 21(3), 206–223.
Kookana, R. S., Aylmore, L. A. G., & Gerritse, R. G. (1992). Time-dependent sorption of pesticides during transport in soils. Soil Science, 154(3), 214–225.
Kronvang, B., Rubæk, G. H., & Heckrath, G. (2009). International Phosphorus Workshop: Diffuse phosphorus loss to surface water bodies—Risk assessment, mitigation options, and ecological effects in river basins. Journal of Environmental Quality, 38(5), 1924–1929. doi:10.2134/jeq2009.0051.
Limousin, G., Gaudet, J. P., Charlet, L., Szenknect, S., Barthes, V., & Krimissa, M. (2007). Sorption isotherms: a review on physical bases, modeling and measurement. Applied Geochemistry, 22(2), 249–275. doi:10.1016/j.apgeochem.2006.09.010.
Lyngsie, G., Borggaard, O. K., & Hansen, H. C. B. (2014a). A three-step test of phosphate sorption efficiency of potential agricultural drainage filter materials. Water Research, 51, 256–265.
Lyngsie, G., Penn, C. J., Hansen, H. C., & Borggaard, O. K. (2014b). Phosphate sorption by three potential filter materials as assessed by isothermal titration calorimetry. Journal of Environmental Management, 143, 26–33.
Makris, K. C., El-Shall, H., Harris, W. G., O’Connor, G. A., & Obreza, T. A. (2004). Intraparticle phosphorus diffusion in a drinking water treatment residual at room temperature. Journal of Colloid and Interface Science, 277(2), 417–423. doi:10.1016/j.jcis.2004.05.001.
McDowell, R. W. (2008). Phosphorus in humped and hollowed soils of the Inchbonnie catchment, West Coast, New Zealand: II. Accounting for losses by different pathways. New Zealand Journal of Agricultural Research, 51(3), 307–316. doi:10.1080/00288230809510462.
McDowell, R. W., & Sharpley, A. N. (2001). Phosphorus losses in subsurface flow before and after manure application to intensively farmed land. Science of the Total Environment, 278(1), 113–125. doi:10.1016/S0048-9697(00)00891-3.
Murphy, J. A. M. E. S., & Riley, J. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36.
Nooney, M. G., Campbell, A., Murrell, T. S., Lin, X. F., Hossner, L. R., Chusuei, C. C., & Goodman, D. W. (1998). Nucleation and growth of phosphate on metal oxide thin films. Langmuir, 14(10), 2750–2755. doi:10.1021/la9702695.
Parfitt, P. L. (1978). Anion adsorption by soils and soil materials. Advances in Agronomy, 30, 1–50.
Penn, C. J., Bryant, R. B., Kleinman, P. J., & Allen, A. L. (2007). Removing dissolved phosphorus from drainage ditch water with phosphorus sorbing materials. Journal of Soil and Water Conservation, 62(4), 269–276.
Penn, C. J., & McGrath, J. M. (2011). Predicting phosphorus sorption onto steel slag using a flow-through approach with application to a pilot scale system. Journal of Water Resource and Protection, 3(4), 235.
Penn, C. J., McGrath, J. M., Rounds, E., Fox, G., & Heeren, D. (2012). Trapping phosphorus in runoff with a phosphorus removal structure. Journal of Environmental Quality, 41(3), 672–679.
Pote, D. H., Daniel, T. C., Nichols, D., Sharpley, A. N., Moore, P. A., Miller, D. M., & Edwards, D. R. (1999). Relationship between phosphorus levels in three Ultisols and phosphorus concentrations in runoff. Journal of Environmental Quality, 28(1), 170–175. doi:10.2134/jeq1999.00472425002800010020x.
Rao, P. S. C., Rolston, D. E., Jessup, R. E., & Davidson, J. M. (1980). Solute transport in aggregated porous media: Theoretical and experimental evaluation. Soil Science Society of America Journal, 44(6), 1139–1146.
SAS Institute Inc. (2008). SAS/STAT® 9.2. User’s Guide. Cary: SAS Institute Inc.
Schoumans, O. F., Chardon, W. J., Bechmann, M. E., Gascuel-Odoux, C., Hofman, G., Kronvang, B., et al. (2014). Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Science of the Total Environment, 468, 1255–1266. doi:10.1016/j.scitotenv.2013.08.061.
Sharpley, A. N. (1995). Dependence of runoff phosphorus on extractable soil phosphorus. Journal of Environmental Quality, 24(5), 920–926.
Sims, J. T., Simard, R. R., & Joern, B. C. (1998). Phosphorus loss in agricultural drainage: Historical perspective and current research. Journal of Environmental Quality, 27(2), 277–293.
Sposito, G. (1980). Derivation of the Freundlich equation for ion exchange reactions in soils. Soil Science Society of America Journal, 44(3), 652–654. doi:10.2136/sssaj1980.03615995004400030045x.
Suzuki, T., Inomata, S., & Sawada, K. (1986). Adsorption of phosphate on calcite. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 82(6), 1733–1743. doi:10.1039/F19868201733.
Sø, H. U., Postma, D., Jakobsen, R., & Larsen, F. (2011). Sorption of phosphate onto calcite; results from batch experiments and surface complexation modeling. Geochimica et Cosmochimica Acta, 75(10), 2911–2923. doi:10.1016/j.gca.2011.02.031.
Søndergaard, M., Jeppesen, E., Peder Jensen, J., & Lildal Amsinck, S. (2005). Water Framework Directive: ecological classification of Danish lakes. Journal of Applied Ecology, 42, 616–629. doi:10.1111/j.1365-2664.2005.01040.x.
Vadas, P. A., Good, L. W., Moore, P. A., & Widman, N. (2009). Estimating phosphorus loss in runoff from manure and fertilizer for a phosphorus loss quantification tool. Journal of Environmental Quality, 38(4), 1645–1653. doi:10.2134/jeq2008.0337.
Van Genuchten, M. T., Wierenga, P. J., & O’connor, G. A. (1977). Mass transfer studies in sorbing porous media: III. Experimental evaluation with 2, 4, 5-T. Soil Science Society of America Journal, 41(2), 278–285. doi:10.2136/sssaj1977.03615995004100020023x.
Van Riemsdijk, W. H., Boumans, L. J. M., & De Haan, F. A. M. (1984). Phosphate sorption by soils: I. A model for phosphate reaction with metal-oxides in soil. Soil Science Society of America Journal, 48(3), 537–541.
Van Riemsdijk, W. H., & Lyklema, J. (1980). The reaction of phosphate with aluminum hydroxide in relation with phosphate bonding in soils. Colloids and Surfaces, 1(1), 33–44. doi:10.1016/0166-6622(80)80036-9.
Veith, J. A., & Sposito, G. (1977). On the use of the Langmuir equation in the interpretation of “adsorption” phenomena. Soil Science Society of America Journal, 41(4), 697–702. doi:10.2136/sssaj1977.03615995004100040015x.
Vohla, C., Kõiv, M., Bavor, H. J., Chazarenc, F., & Mander, Ü. (2011). Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecological Engineering, 37(1), 70–89. doi:10.1016/j.ecoleng.2009.08.003.
Westholm, L. J. (2006). Substrates for phosphorus removal—Potential benefits for on-site wastewater treatment? Water Research, 40(1), 23–36.
Willett, I. R., Chartres, C. J., & Nguyen, T. T. (1988). Migration of phosphate into aggregated particles of ferrihydrite. Journal of Soil Science, 39(2), 275–282. doi:10.1111/j.1365-2389.1988.tb01214.x.
Wood, W. W., Kraemer, T. F., & Hearn, P. P. (1990). Intragranular diffusion: An important mechanism influencing solute transport in clastic aquifers? Science, 247(4950), 1569–1572. doi:10.1126/science.247.4950.1569.
Wu, T., & Sansalone, J. (2013). Phosphorus equilibrium. I: Impact of AlOx media substrates and aqueous matrices. Journal of Environmental Engineering, 139(11), 1315–1324.
Yagi, S., & Fukushi, K. (2012). Removal of phosphate from solution by adsorption and precipitation of calcium phosphate onto monohydrocalcite. Journal of Colloid and Interface Science, 384(1), 128–136. doi:10.1016/j.jcis.2012.06.063.
Acknowledgments
This research study was part of the project “Sustainable Phosphorus Removal and Recycle Technologies” (Supreme-Tech, www.supremetech.dk) funded by Danish Strategic Research Council, grant number 09-067280. We thank Lene Skovmose Andersen and Michael Koppelgaard for their technical assistance in laboratory during construction of the column setup and Kristian Kristensen for his suggestions on statistical issues.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canga, E., Heckrath, G.J. & Kjaergaard, C. Agricultural Drainage Filters. II. Phosphorus Retention and Release at Different Flow Rates. Water Air Soil Pollut 227, 276 (2016). https://doi.org/10.1007/s11270-016-2963-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2963-3