Abstract
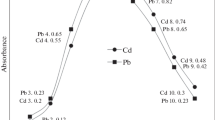
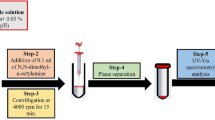
This is a green method for determination of mercury ion (Hg2+) in environmental samples. The method of exchangeable water based on liquid-liquid microextraction (EW-LLME) was first time introduced as a green analytical separation technique. Exchangeable water was made by the reaction of carbon dioxide with diethylenetriamine. The exchanging phenomena from low polarity to high polarity were confirmed by Fourier transforms infrared spectrometry. The complex formation between Hg2+ and 1, 5-diphenylcarbazone was achieved under the optimized experimental conditions. The enrichment factor and limits of detection of the present method were obtained to be 45.2 and 0.5 ng L−1, respectively. The accuracy of the present method was confirmed with certified reference materials. The EW-LLME was successfully applied for determination of Hg2+ in solid matrices of block-III and V of Thar coalfield.





Similar content being viewed by others
References
Afkhami, A., Madrakian, T., & Siampour, H. (2006). Highly selective determination of trace quantities of mercury in water samples after preconcentration by the cloud-point extraction method. International Journal of Environmental and Analytical Chemistry, 86(15), 1165–1173.
Ahmad, S., & Chaudhry, M. (2007). Geochemical characterization and origin of the Karai-gabbro from the Neoproterozoic Nagarparker complex, Pakistan. Geol Bull Punjab Univ, 42, 1–14.
Ali, J., Kazi, T. G., Baig, J. A., Afridi, H. I., Arain, M. S., Brahman, K. D., & Panhwar, A. H. (2015a). Arsenic in coal of the Thar coalfield, Pakistan, and its behavior during combustion. Environmental Science and Pollution Research, 22(11), 8559–8566.
Ali, J., Kazi, T. G., Baig, J. A., Afridi, H. I., Arain, M. S., Ullah, N., Arain, S. S., & Siraj, S. (2015b). Monitoring of arsenic fate with proximate parameters and elemental composition of coal from Thar coalfield, Pakistan. Journal of Geochemical Exploration, 159, 227–233.
Ali, J., Kazi, T. G., Baig, J. A., Afridi, H. I., Arain, M. S., Ullah, N., Brahman, K. D., Arain, S. S., & Panhwar, A. H. (2015c). Evaluation of the fate of arsenic-contaminated groundwater at different aquifers of Thar coalfield, Pakistan. Environmental Science and Pollution Research, 22(23), 19251–19263.
Ali, J., Kazi, T. G., Afridi, H. I., Baig, J. A., Arain, M. S., & Farooq, S. (2016). The evaluation of sequentially extracted mercury fractions in Thar coal samples by using different extraction schemes. International Journal of Coal Geology, 156, 50–58.
Álvarez-Ayuso, E., Querol, X., Plana, F., Alastuey, A., Moreno, N., Izquierdo, M., Font, O., Moreno, T., Diez, S., & Vázquez, E. (2008). Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal combustion fly ashes. Journal of Hazardous Materials, 154(1), 175–183.
Arain, M. S., Kazi, T. G., Afridi, H. I., Arain, S. A., Ali, J., Arain, S. S., Panhwar, A. H., & Shanker, B. (2014). Preconcentration and determination of manganese in biological samples by dual-cloud point extraction coupled with flame atomic absorption spectrometry. Journal of Analytical Atomic Spectrometry, 29(12), 2349–2355.
Arain, M. S., Arain, S. A., Kazi, T. G., Afridi, H. I., Ali, J., Arain, S. S., Brahman, K. D., & Mughal, M. A. (2015). Temperature controlled ionic liquid-based dispersive micro-extraction using two ligands, for determination of aluminium in scalp hair samples of Alzheimer’s patients: A multivariate study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137, 877–885.
Armenta, S., Garrigues, S., & Guardia, M. (2008). Green analytical chemistry. Trac Trends in Analytical Chemistry, 27(6), 497–511.
Beach, E. S., Cui, Z., & Anastas, P. T. (2009). Green Chemistry: a design framework for sustainability. Energy & Environmental Science, 2(10), 1038–1049.
Cunha, R. C., Patrício, P. R., Vargas, S. J. R., da Silva, L. H. M., & da Silva, M. C. H. (2016). Green recovery of mercury from domestic and industrial waste. Journal of Hazardous Materials, 304, 417–424.
Dadfarnia, S., Salmanzadeh, A. M., Haji, S., & Ali, M. (2002). Prenconcentration and determination of mercury (II) and methylmercury in waters by immobilized 1, 5-diphenylcarbazone and cold vapor atomic absorption spectrometry. Bulletin of the Korean Chemical Society, 23(12), 1719–1723.
Demirel, S., Tuzen, M., Saracoglu, S., & Soylak, M. (2008). Evaluation of various digestion procedures for trace element contents of some food materials. Journal of Hazardous Materials, 152(3), 1020–1026.
Dutta, S., Mathews, R. P., Singh, B. D., Tripathi, S. M., Singh, A., Saraswati, P. K., Banerjee, S., & Mann, U. (2011). Petrology, palynology and organic geochemistry of Eocene lignite of Matanomadh, Kutch Basin, western India: Implications to depositional environment and hydrocarbon source potential. International Journal of Coal Geology, 85(1), 91–102.
Flores, É. M., Welz, B., & Curtius, A. J. (2001). Determination of mercury in mineral coal using cold vapor generation directly from slurries, trapping in a graphite tube, and electrothermal atomization. Spectrochimica Acta Part B: Atomic Spectroscopy, 56(9), 1605–1614.
Fontas, C., Hidalgo, M., Salvadó, V., & Antico, E. (2005). Selective recovery and preconcentration of mercury with a benzoylthiourea-solid supported liquid membrane system. Analytica Chimica Acta, 547(2), 255–261.
Ghaedi, M., Reza Fathi, M., Shokrollahi, A., & Shajarat, F. (2006). Highly selective and sensitive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy. Analytical Letters, 39(6), 1171–1185.
Guo, H., Zhou, Z., & Jing, G. (2013). Kinetics of carbon dioxide absorption into aqueous [Hmim][Gly] solution. International Journal of Greenhouse Gas Control, 16, 197–205.
Hakim, L., Sabarudin, A., Oshima, M., & Motomizu, S. (2007). Synthesis of novel chitosan resin derivatized with serine diacetic acid moiety and its application to on-line collection/concentration of trace elements and their determination using inductively coupled plasma-atomic emission spectrometry. Analytica Chimica Acta, 588(1), 73–81.
Han, F. X., Su, Y., Monts, D. L., Waggoner, C. A., & Plodinec, M. J. (2006). Binding distribution and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Science of the Total Environment, 368(2), 753–768.
Heldebrant, D. J., Yonker, C. R., Jessop, P. G., & Phan, L. (2008). Organic liquid CO2 capture agents with high gravimetric CO2 capacity. Energy & Environmental Science, 1(4), 487–493.
Imran, M., Kumar, D., Kumar, N., Qayyum, A., Saeed, A., & Bhatti, M. S. (2014). Environmental concerns of underground coal gasification. Renewable and Sustainable Energy Reviews, 31, 600–610.
Jessop, P. G., & Subramaniam, B. (2007). Gas-expanded liquids. Chemical Reviews, 107(6), 2666–2694.
Jiménez-González, C., Poechlauer, P., Broxterman, Q. B., Yang, B.-S., Am Ende, D., Baird, J., Bertsch, C., Hannah, R. E., Dell’Orco, P., & Noorman, H. (2011). Key green engineering research areas for sustainable manufacturing: A perspective from pharmaceutical and fine chemicals manufacturers. Organic Process Research & Development, 15(4), 900–911.
Kilaru, P. K., & Scovazzo, P. (2008). Correlations of low-pressure carbon dioxide and hydrocarbon solubilities in imidazolium phosphonium and ammonium based room temperature ionic liquids. Part 2. Using activation energy of viscosity. Industrial & Engineering Chemistry Research, 47(3), 910–919.
Kirchner, B., Stubbs, J., & Marx, D. (2002). Fast anomalous diffusion of small hydrophobic species in water. Physical Review Letters, 89(21), 215901.
Knöfel, C., Martin, C., Hornebecq, V., & Llewellyn, P. L. (2009). Study of carbon dioxide adsorption on mesoporous aminopropylsilane-functionalized silica and titania combining microcalorimetry and in situ infrared spectroscopy. The Journal of Physical Chemistry, 113(52), 21726–21734.
Lestari, G., Abolhasani, M., Bennett, D., Chase, P., Günther, A., & Kumacheva, E. (2014). Switchable water: microfluidic investigation of liquid–liquid phase separation mediated by carbon dioxide. Journal of the American Chemical Society, 136(34), 11972–11979.
Li, Z., Xia, S., Wang, J., Bian, C., & Tong, J. (2016). Determination of trace mercury in water based on N-octylpyridinium ionic liquids preconcentration and stripping voltammetry. Journal of Hazardous Materials, 301, 206–213.
López-Antón, M. A., Díaz-Somoano, M., Ochoa-González, R., & Martínez-Tarazona, M. R. (2012). Analytical methods for mercury analysis in coal and coal combustion byproducts. International Journal of Coal Geology, 94, 44–53.
Lv, B., Guo, B., Zhou, Z., & Jing, G. (2015). Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environmental Science & Technology, 49(17), 10728–10735.
Machnikowska, H., Krztoń, A., & Machnikowski, J. (2002). The characterization of coal macerals by diffuse reflectance infrared spectroscopy. Fuel, 81(2), 245–252.
Mahpishanian, S., & Shemirani, F. (2010). Preconcentration procedure using in situ solvent formation microextraction in the presence of ionic liquid for cadmium determination in saline samples by flame atomic absorption spectrometry. Talanta, 82(2), 471–476.
Malkani, M. S. (2012). A review of coal and water resources of Pakistan. Journal of Science, Technology and Development, 31(3), 202–218.
Mercer, S. M., & Jessop, P. G. (2010). Switchable water: Aqueous solutions of switchable ionic strength. ChemSusChem, 3(4), 467–470.
Mercer, S. M., Robert, T., Dixon, D. V., Chen, C. S., Ghoshouni, Z., Harjani, J. R., Jahangiri, S., Peslherbe, G. H., & Jessop, P. G. (2012). Design, synthesis, and solution behaviour of small polyamines as switchable water additives. Green Chemistry, 14(3), 832–839.
Monalisa, K. A., & Jan, M. Q. (2007). Seismic hazard assessment of the NW Himalayan fold and thrust belt, Pakistan, using probabilistic approach. Journal of Earthquake Engineering, 11(2), 257–301.
Naseem, S., Rafique, T., Bashir, E., Bhanger, M. I., Laghari, A., & Usmani, T. H. (2010). Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere, 78(11), 1313–1321.
Naseri, M. T., Hemmatkhah, P., Hosseini, M. R. M., & Assadi, Y. (2008). Combination of dispersive liquid–liquid microextraction with flame atomic absorption spectrometry using microsample introduction for determination of lead in water samples. Analytica Chimica Acta, 610(1), 135–141.
Phan, L., Brown, H., White, J., Hodgson, A., & Jessop, P. G. (2009). Soybean oil extraction and separation using switchable or expanded solvents. Green Chemistry, 11(1), 53–59.
Rofouei, M. K., Hosseini, S. M., Khani, H., & Rahimi-Alangi, S. (2012). Highly selective determination of trace quantities of Hg (ii) in water samples by spectrophotometric and inductively coupled plasma-optical emission spectrometry methods after cloud point extraction. Analytical Methods, 4(3), 759–765.
Shah, A., Kazi, T., Baig, J., Afridi, H., Kandhro, G., Arain, M., Kolachi, N., & Wadhwa, S. (2010). Total mercury determination in different tissues of broiler chicken by using cloud point extraction and cold vapor atomic absorption spectrometry. Food and Chemical Toxicology, 48(1), 65–69.
Singhvi, A., & Kar, A. (2004). The aeolian sedimentation record of the Thar Desert. Journal of Earth System Science, 113(3), 371–401.
Smith, R. M. (2006). Superheated water: the ultimate green solvent for separation science. Analytical and Bioanalytical Chemistry, 385(3), 419–421.
Su, X., Cunningham, M. F., & Jessop, P. G. (2014). Use of a switchable hydrophobic associative polymer to create an aqueous solution of CO2 switchable viscosity. Polymer Chemistry, 5(3), 940–944.
Sun, J., Li, S. H., Han, P., & Chen, Y. (2006). Holocene environmental changes in the central Inner Mongolia, based on single aliquot quartz optical dating and multi-proxy study of dune sands. Palaeogeography Palaeoclimatology Palaeoecology, 233(1), 51–62.
Sun, H., Zhou, X. Q., Xue, Z., Zhou, Z. Y., & Mu, T. (2014). Theoretical investigations on the reaction mechanisms of amine-functionalized ionic liquid [aEMMIM][BF 4] and CO2. International Journal of Greenhouse Gas Control, 20, 43–48.
Yu, J., Le, Y., & Cheng, B. (2012). Fabrication and CO2 adsorption performance of bimodal porous silica hollow spheres with amine-modified surfaces. RSC Advances, 2(17), 6784–6791.
Zhang, C., Huang, K., Yu, P., & Liu, H. (2011). Salting-out induced three liquid phase separation of Pt (IV), Pd (II) and Rh (III) in system of S201 acetonitrile NaCl water. Separation and Purification Technology, 80(1), 81–89.
Acknowledgments
Jamshed Ali is grateful to the Scientific and Technological Research Council of Turkey TUBITAK-BIDEB 2216 Research Fellowship Programme for International Researchers for providing financial support. The authors also would like to thank to Gaziosmanpasa, University. Dr. Mustafa Tuzen thanks theTurkish Academy of Sciences for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, J., Tuzen, M. & Kazi, T.G. Determination of Mercury in Environmental Samples by Using Water Exchangeable Liquid-Liquid Microextraction as Green Extraction Method Couple with Cold Vapor Technique. Water Air Soil Pollut 227, 170 (2016). https://doi.org/10.1007/s11270-016-2863-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2863-6