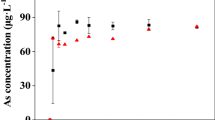
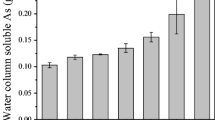
Abstract
Effects of pH, As species, and Fe/Mn minerals on the fractions of adsorbed As in aquifer sediments were evaluated. Kinetic data showed that As adsorption was controlled by diffusion through the external film. Isothermal data of both As(III) and As(V) fitted the Langmuir isotherm well, revealing a monolayer adsorption process. Sequential extraction demonstrated that water-soluble As and non-specifically sorbed As were the major fractions of adsorbed As. Assessing the relationship between the Freundlich K F and the increases in the amounts of As fractions showed that the pH played a key role in weakly adsorbed As, especially water-soluble As. Although inorganic As species converted each other during the adsorption processes, more non-specifically sorbed As was adsorbed in As(V)-treated sediment than in As(III)-treated sediment, showing that the electrostatic selectivity controlled the non-specific adsorption. Additionally, specifically sorbed As and As associated with the amorphous phases were predominated by Fe/Mn minerals, especially Fe(III) (hydr)oxides. These results suggested that pH, As species, and Fe/Mn minerals would regulate the As fractions in aquifer sediments, and therefore control As cycling in aquifer systems.






Similar content being viewed by others
References
Amstaetter, K., Borch, T., Karese-casanova, P., & Kappler, A. (2010). Redox transformation of arsenic by Fe(II)-activated goethite (α-FeOOH). Environmental Science & Technology, 44(1), 102–108.
Anawar, H. M., Akai, J., & Sakugawa, H. (2004). Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere, 54(6), 753–762.
Asta, M. P., Cama, J., Martínez, M., & Giménez, J. (2009). Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. Journal of Hazardous Materials, 171(1–3), 965–972.
Banerjee, K., Amy, G. L., Prevost, M., Nour, S., Jekel, M., Gallagher, P. M., & Blumenschein, C. D. (2008). Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Research, 42(13), 3371–3378.
Campbell, K. M., Malasarn, D., Saltikov, C. W., Newman, D. K., & Hering, J. G. (2006). Simultaneous microbial reduction of iron (III) and arsenic (V) in suspensions of hydrous ferric oxide. Environmental Science & Technology, 40(19), 5950–5955.
Chang, M. Y., & Juang, R. S. (2004). Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. Journal of Colloid and Interface Science, 278(1), 18–25.
Costagliola, P., Bardelli, F., Benvenuti, M., Benedetto, F. D., Lattanzi, P., Romanelli, M., Paolieri, M., Rimondi, V., & Vaggelli, G. (2013). Arsenic-bearing calcite in natural travertines: evidence from sequential extraction, μXAS, and μXRF. Environmental Science & Technology, 47(12), 6231–6238.
Deschamps, E., Ciminelli, V. S. T., Weidler, P. G., & Ramos, A. Y. (2003). Arsenic sorption onto soils enriched in Mn and Fe minerals. Clays and Clay Minerals, 51(2), 197–204.
Devesa-Rey, R., Paradelo, R., Díaz-Fierros, F., & Barral, M. T. (2008). Fractionation and bioavailability of arsenic in the bed sediments of the Anllóns River (NW Spain). Water, Air, & Soil Pollution, 195(1), 189–198.
Doušová, B., Martaus, A., Filippi, M., & Koloušek, D. (2008). Stability of arsenic species in soils contaminated naturally and in an anthropogenic manner. Water, Air, & Soil Pollution, 187(1–4), 233–241.
Fendorf, S., Eick, M. J., Grossl, P., & Sparks, D. L. (1997). Arsenate and chromate retention mechanisms on goethite. 1. Surface structure. Environmental Science & Technology, 31(2), 315–320.
Fendorf, S., Michael, H. A., & van Geen, A. (2010). Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science, 328(5982), 1123–1127.
Fritzsche, A., Rennert, T., & Totsche, K. U. (2011). Arsenic strongly associates with ferrihydrite colloids formed in a soil effluent. Environmental Pollution, 159(5), 1398–1405.
Gao, X. B., Su, C. L., Wang, Y. X., & Hu, Q. H. (2013). Mobility of arsenic in aquifer sediments at Datong Basin, northern China: effect of bicarbonate and phosphate. Journal of Geochemical Exploration, 135(5), 93–103.
Giral, M., Zagury, G. J., Deschênes, L., & Blouin, J. (2010). Comparison of four extraction procedures to assess arsenate and arsenite species in contaminated soils. Environmental Pollution, 158(5), 1890–1898.
Goldberg, S., & Johnstonm, C. T. (2001). Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modelling. Journal of Colloid and Interface Science, 234(1), 204–216.
Gunduz, O., Simsek, C., & Hasozbek, A. (2010). Arsenic pollution in the groundwater of Simav Plain, Turkey: its impact on water quality and human health. Water, Air, & Soil Pollution, 205(1), 43–62.
Guo, H. M., Stüben, D., & Berner, Z. (2007). Adsorption of arsenic(III) and arsenic(V) from groundwater using natural siderite as the adsorbent. Journal of Colloid and Interface Science, 315(1), 47–53.
Guo, H. M., Li, Y., & Zhao, K. (2010). Arsenate removal from aqueous solution using synthetic siderite. Journal of Hazardous Materials, 176(1), 174–180.
Guo, H. M., Ren, Y., Liu, Q., Zhao, K., & Li, Y. (2013). Enhancement of arsenic adsorption during mineral transformation from siderite to goethite: mechanism and application. Environmental Science & Technology, 47(2), 1009–1016.
Guo, H. M., Wen, D. G., Liu, Z. Y., Jia, Y. F., & Guo, Q. (2014). A review of high arsenic groundwater in Mainland and Taiwan, China: Distribution, characteristics and geochemical processes. Applied Geochemistry, 41, 196–217.
Gupta, V. K., Saini, V. K., & Jain, N. (2005). Adsorption of As(III) from aqueous solutions by iron oxide-coated sand. Journal of Colloid and Interface Science, 288(1), 55–60.
Han, F. X., Kingery, W. L., Selim, H. M., Gerard, P. D., Cox, M. S., & Oldham, J. L. (2004). Arsenic solubility and distribution in poultrywaste and long-term amended soil. Science of the Total Environment, 320(1), 51–61.
Ho, Y. S. (2004). Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics, 59(1), 171–177.
Ho, Y. S., & Mckay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465.
Huang, G. X., Chen, Z. Y., Wang, J. C., Sun, J. C., Liu, J. T., & Zhang, Y. (2013). Adsorption of arsenite onto a soil irrigated by sewage. Journal of Geochemical Exploration, 132, 164–172.
Huang, G. X., Chen, Z. Y., Sun, J. C., Liu, F., Wang, J., & Zhang, Y. (2015). Effect of sample pretreatment on the fractionation of arsenic in anoxic soils. Environmental Science and Pollution Research, 22(11), 8367–8374.
Hug, S. J., & Leupin, O. (2003). Iron-catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction. Environmental Science & Technology, 37(12), 2734–2742.
Islam, F. S., Gault, A. G., Boothman, C., Polya, D. A., Charnock, J. M., Chatterjee, D., & Lloyd, J. R. (2004). Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature, 430(6995), 68–71.
Javed, M. B., Kachanoski, G., & Siddique, T. (2013). A modified sequential extraction method for arsenic fractionation in sediments. Analytica Chimica Acta, 787(17), 102–110.
Jessen, S., Larsen, F., Koch, C. B., & Avin, E. (2005). Sorption and desorption of arsenic to ferrihydrite in a sand filter. Environmental Science & Technology, 39(20), 8045–8051.
Katsoyiannis, I. A., Ruettimann, T., & Hug, S. J. (2008). pH dependence of Fenton reagent generation and As(III) oxidation and removal by corrosion of zero valent iron in aerated water. Environmental Science & Technology, 42(19), 7424–7430.
Keon, N. E., Swartz, C. H., Brabander, D. J., Harveyand, C., & Hemond, H. F. (2001). Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environmental Science & Technology, 35(13), 2778–2784.
Kim, E. J., Yoo, J., & Baek, K. (2014). Arsenic speciation and bioaccessibility in arsenic-contaminated soils: sequential extraction and mineralogical investigation. Environmental Pollution, 186, 29–35.
Kosmulski, M. (2009). Compilation of PZC and IEP of sparingly soluble metal oxides and hydroxides from literature. Advances in Colloid and Interface Science, 152(1), 14–25.
Kumar, K. V., Ramamurthi, V., & Sivanesan, S. (2005). Modeling the mechanism involved during the sorption of methylene blue onto fly ash. Journal of Colloid and Interface Science, 284(1), 14–21.
Lenz, C., Behrends, T., Jilbert, T., Silveira, M., & Slomp, C. P. (2014). Redox-dependent changes in manganese speciation in Baltic Sea sediments from the Holocene Thermal Maximum: An EXAFS, XANES and LA-ICP-MS study. Chemical Geology, 370(26), 49–57.
Liang, Q. Q., & Zhao, D. Y. (2014). Immobilization of arsenate in a sandy loam soil using starch-stabilized magnetite nanoparticles. Journal of Hazardous Materials, 271(30), 16–23.
Livesey, N. T., & Huang, P. M. (1981). Adsorption of arsenate by soils and its relation to selected chemical properties and anions. Soil Science, 131(2), 88–94.
Maji, S. K., Pal, A., Pal, T., & Adak, A. (2007). Adsorption thermodynamics of arsenic on laterite soil. Journal of Surface Science and Technology, 22(3–4), 161–176.
Mamindy-Pajany, Y., Hurel, C., Marmier, N., & Roméo, M. (2011). Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: effects of pH, concentration and reversibility. Desalination, 281(17), 93–99.
Manning, B. A., Fendorf, S. E., & Goldberg, S. (1998). Surface structures and stability of arsenic(III) on goethite: spectroscopic evidence for inner-sphere complexes. Environmental Science & Technology, 32(16), 2383–2388.
Manning, B. A., Fendorf, S. E., Bostick, B., & Suarez, D. L. (2002a). Arsenic(III) oxidation and arsenic(V) adsorption reactions on synthetic birnessite. Environmental Science & Technology, 36(5), 976–981.
Manning, B. A., Hunt, M. L., Amrhein, C., & Yarmoff, J. A. (2002b). Arsenic(III) and arsenic(V) reactions with zero valent iron corrosion products. Environmental Science & Technology, 36(24), 5455–5461.
Mohan, D., & Pittman, C. U., Jr. (2007). Arsenic removal from water/wastewater using adsorbents—a critical review. Journal of Hazardous Materials, 142(1–2), 1–53.
Mukherjee, A., Scanlon, B. R., Fryar, A. E., Saha, D., Ghosh, A., Chowdhuri, S., & Mishra, R. (2012). Solute chemistry and arsenic fate in aquifers between the Himalayan foothills and Indian craton (including central Gangetic plain): influence of geology and geomorphology. Geochimica et Cosmochimica Acta, 90(1), 283–302.
Namasivayam, C., & Senthilkumar, S. (1998). Removal of arsenic(V) from aqueous solution using industrial solid waste: adsorption rates and equilibrium studies. Industrial & Engineering Chemistry Research, 37(12), 4816–4822.
Nath, B., Sahu, S. J., Jana, J., Mukherjee-Goswami, A., Roy, S., & Sarkar, M. J. (2008). Hydrochemistry of arsenic-enriched aquifer from rural West Bengal, India: a study of the arsenic exposure and mitigation option. Water, Air, & Soil Pollution, 190(1–4), 95–113.
Nesbitt, H. W., Canning, G. W., & Bancroft, G. M. (1998). XPS study of reductive dissolution of 7 Å-birnessite by H3AsO3, with constraints on reaction mechanism. Geochimica et Cosmochimica Acta, 62(12), 2097–2110.
Ona-Nguema, G., Morin, G., Wang, Y. H., Foster, A. L., Juillot, F., Calas, G., & Brown, G. E. (2010). XANES evidence for rapid arsenic(III) oxidation at magnetite and ferrihydrite surfaces by dissolved O2 via Fe2+-mediated reactions. Environmental Science & Technology, 44(14), 5416–5422.
Oscarson, D. W., Huang, P. M., & Liaw, W. K. (1981). Role of manganese in the oxidation of arsenite by freshwater lake sediments. Clays and Clay Minerals, 29(3), 219–225.
Osorio-López, C., Seco-Reigosa, N., Garrido-Rodríguez, B., Cutillas-Barreiro, L., Arias-Estévez, M., Fernández-Sanjurjo, M. J., Álvarez-Rodríguez, E., & Núñez-Delgado, A. (2014). As(V) adsorption on forest and vineyard soils and pyritic material with or without mussel shell: kinetics and fractionation. Journal of the Taiwan Institute of Chemical Engineers, 45(3), 1007–1014.
Partey, F., Norman, D., Ndur, S., & Nartey, R. (2008). Arsenic sorption onto laterite iron concretions: temperature effect. Journal of Surface Science and Technology, 321(2), 493–500.
Poulton, S. W., & Canfield, D. E. (2005). Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates. Chemical Geology, 214(3–4), 209–221.
Qi, Y. Q., & Donahoe, R. J. (2008). The environmental fate of arsenic in surface soil contaminated by historical herbicide application. Science of the Total Environment, 405(1–3), 246–254.
Qiao, J. L., Jiang, Z., Sun, B., Sun, Y. K., Wang, Q., & Guan, X. H. (2012). Arsenate and arsenite removal by FeCl3: effects of pH, As/Fe ratio, initial As concentration and co-existing solutes. Separation and Purification Technology, 92(18), 106–114.
Ramesh, A., Hasegawa, H., Maki, T., & Ueda, K. (2007). Adsorption of inorganic and organic arsenic from aqueous solutions by polymeric Al/Fe modified montmorillonite. Separation and Purification Technology, 56(1), 90–100.
Ravenscroft, P., Brammer, H., & Richards, K. (2009). Arsenic pollution: a global synthesis. Oxford: Wiley-Blackwell.
Robert, L. C., Hug, S. J., Ruettimann, T., Billah, M. M., Khan, A. W., & Rahman, M. T. (2004). Arsenic removal with iron(II) and iron(III) in waters with high silicate and phosphate concentrations. Environmental Science & Technology, 38(1), 307–315.
Salameh, Y., Al-Lagtah, N., Ahmad, M. N. M., Allen, S. J., & Walker, G. M. (2010). Kinetic and thermodynamic investigations on arsenic adsorption onto dolomitic sorbents. Chemical Engineering Journal, 160(2), 440–446.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568.
Stanic, T., Dakovic, A., Zivanovic, A., Tomasevic-Canovic, M., Dondur, V., & Milicevic, S. (2009). Adsorption of arsenic(V) by iron III-modified natural zeolitic tuff. Environmental Chemistry Letters, 7(2), 161–166.
Teppen, B. J., Yu, C. H., Newton, S. Q., Miller, D. M., & Schtifer, L. (1998). Ab initio investigations pertaining to aluminum in tetrahedral octahedral sites of clay minerals. Journal of Molecular Structure, 445(1–3), 65–88.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Tufano, K. J., Reyes, C., Saltikov, C. W., & Fendof, S. (2008). Reductive processes controlling arsenic retention: revealing the relative importance of iron and arsenic reduction. Environmental Science & Technology, 42(22), 8283–8289.
Wenzel, W. W., Kirchbaumer, N., Prohaska, T., Stingeder, G., Lombi, E., & Adriano, D. C. (2001). Arsenic fractionation in soils using an improved sequential extraction procedure. Analytica Chimica Acta, 436(2), 309–323.
Ying, C., Kocar, B. D., & Fendorf, S. (2012). Oxidation and competitive retention of arsenic between iron- and manganese oxides Samantha. Geochimica et Cosmochimica Acta, 96(1), 294–303.
Zhang, H., & Selim, H. M. (2005). Kinetics of arsenate adsorption-desorption in soils. Environmental Science & Technology, 39(16), 6101–6108.
Acknowledgments
The study is financially supported by the National Natural Science Foundation of China (Nos. 41222020, 41172224, and 41271479), the Fundamental Research Funds for the Central Universities (No. 2652013028), and the Fok Ying-Tung Education Foundation, China (Grant No. 131017), and Equipment Function Development and Technology Innovation projects of Chinese Academy of Sciences (No. Y3R30020TS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, J., Guo, H., Lei, M. et al. Arsenic Adsorption and its Fractions on Aquifer Sediment: Effect of pH, Arsenic Species, and Iron/Manganese Minerals. Water Air Soil Pollut 226, 260 (2015). https://doi.org/10.1007/s11270-015-2524-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2524-1