Abstract
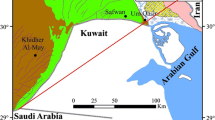
Tungsten (W) concentrations were measured along with arsenic (As) in groundwaters from the Murshidabad district of West Bengal, India. Tungsten concentrations range from 0.8 to ~8 nmol kg-1 (0.15–1.5 μg kg-1) in the circumneutral pH (average pH ~ 7.3) Murshidabad groundwaters, and attain concentrations as high as 14 nmol kg-1 (2.5 μg kg-1) in local ponds (n = 2). Total dissolved As concentrations (AsT) range from 0.013 to 53.9 μmol kg-1 (<1 to 4,032 μg kg-1), and As(III) predominates in Murshidabad groundwaters accounting for 70 %, on average, of As in solution. Tungsten concentrations in Murshidabad groundwaters are low compared to alkaline groundwaters (pH > 8) from the Carson Desert in Western Nevada, USA, where W concentrations are reported to reach as high as 4,036 nmol kg-1 (742 μg kg-1). Although W is positively correlated with As in groundwaters from the Carson Desert, it is not correlated with AsT or As(III) in Murshidabad groundwaters, but does exhibit a weak relationship with As(V) in these groundwaters. Surface complexation modeling indicates that pH related adsorption/desorption can explain the geochemical behavior of W in Murshidabad groundwaters. However, the model does not predict the high As concentrations observed in Murshidabad groundwaters. The high As and low W concentrations measured in Murshidabad groundwaters indicate that either As and W originate from different sources or are mobilized by different biogeochemical processes within the Murshidabad aquifers. Mobilization of As in Murshidabad groundwaters is presumed to reflect reductive dissolution of Fe(III) oxides/oxyhydroxides and release of sorbed and/or coprecipitated As to the groundwaters. Multivariate statistical analysis of groundwater composition data indicate that W is associated with Mn and Cl-, which may point to a Mn oxide/oxyhydroxide, clay mineral, and/or apatite source for W in the Murshidabad sediments.






Similar content being viewed by others
References
Anderson, T.W. (2003). An introduction to multivariate statistical analysis (3rd ed.). Wiley Series in Probability and Statistics. Wiley–Interscience.
Anderson, T. W., & Darling, D. A. (1954). A test of goodness of fit. Journal of the American Statistical Association, 49, 765–769.
Arnórsson, S., & Óskarsson, N. (2007). Molybdenum and tungsten in volcanic rocks and in surface and <100 °C ground waters in Iceland. Geochimica et Cosmochimica Acta, 71, 284–304.
ATSDR–Agency for Toxic Substances and Disease Registry. (2005). Toxicological Profile for Tungsten. http://www.atsdr.cdc.gov/toxprofiles/tp186.pdf. Accessed 3 December 2009.
Baes, C. F., Jr., & Mesmer, R. E. (1976). The hydrolysis of cations. New York: Wiley.
Bednar, A. J., Mirecki, J. E., Inouye, L. S., Winfield, L. E., Larson, S. L., & Ringelberg, D. B. (2007). The determination of tungsten, molybdenum, and phosphorus oxyanions by high performance liquid chromatography inductively coupled plasma mass spectrometry. Talanta, 72, 1828–1832.
Bednar, A. J., Jones, W. T., Boyd, R. E., Ringelberg, D. C., & Larson, S. L. (2008). Geochemical parameters influencing tungsten mobility in soils. Journal of Environmental Quality, 37, 229–233.
Bednar, A. J., Boyde, R. E., Jones, W. T., McGrath, C. J., Johnson, D. R., Chappell, M. A., et al. (2009). Investigation of tungsten mobility in soil using column tests. Chemosphere, 75, 1049–1056.
Bethke, C. M. (2008). Geochemical and biogeochemical reaction modeling (p. 543). Cambridge, UK: Cambridge University Press.
Bethke, C. M., & Yeakel, S. (2010). Geochemist’s Workbench® Release 8.0: Reaction modeling guide (p. 84). Urbana: University of Illinois.
Box, G. E. P., & Cox, D. R. (1964). An analysis of transformations. Journal of the Royal Statistical Society, Series B: Statistical Methodology, 26(2), 211–252.
Canty, A., Ripley, B. (2012). Boot: Bootstrap R (S–Plus) functions, R package version 1.3–4.
Carr, J. R. (1990). CORSPOND: a portable FORTRAN-77 program for correspondence analysis. Computers and Geosciences, 16, 289–307.
Carr, J. R. (1995). Numerical analysis for the geological sciences. Englewood Cliffs, NJ: Prentice-Hall. 592 pp.
Carr, J. R. (2002). Data visualization in the geosciences. Upper Saddle River: Prentice-Hall. 267 pp.
Clausen, J. L., Ketterer, M. E., Bednar, A. J., & Koening, M. R. (2010). Challenges and successes in using inductively coupled plasma mass spectrometry for measurements of tungsten in environmental water and soil samples. International Journal of Environmental Analytical Chemistry, 90, 773–783.
Clausen, J. L., Bostick, B. C., Bednar, A., Sun, J., Landis, J. D. (2011). Tungsten speciation in firing range soils (82 pp.). U.S. Army Corps of Engineers, Engineering Research and Development Center Report TR-11-1.
Cruywagen, J. J., & van der Merwe, I. F. J. (1987). Tungsten(VI) equilibria: A potentiometric and calorimetric investigation. Journal of the Chemical Society, Dalton Transactions, 7, 1701–1705.
Datta, S., Neal, A. W., Mohajerin, T. J., Ocheltree, T., Rosenheim, B. E., White, C. D., et al. (2011). Perennial ponds are not an important source of water or dissolved organic matter to groundwaters with high arsenic concentrations in West Bengal, India. Geophysical Research Letters, 38, L20404. doi:10.1029/2011GL049301.
Davis, J. C. (1986). Statistics and data analysis in geology (2nd ed.). New York: Wiley. 646 pp.
Development Core Team, R. (2012). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. 3-900051-07-0.
DiCiccio, T. J., & Efron, B. (1996). Bootstrap confidence intervals. Statistical Science, 11(3), 189–228.
Dixit, S., & Hering, J. G. (2003). Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environmental Science & Technology, 37, 4182–4189.
Dzombak, D., & Morel, F. M. M. (1990). Surface complexation modeling: Hydrous ferric oxide. New York: Wiley. 393 pp.
Edel, J., Sabbioni, E., Pietra, R., Rossi, A., Torre, M., Rizzato, G., et al. (1990). Trace metal lung disease: In vitro interaction of hard metals with human lung and plasma components. Science of the Total Environment, 95, 107–117.
Farnham, I. M., Singh, A. K., Stetzenbach, K. J., & Johannesson, K. H. (2002). Treatment of nondetects in multivariate analysis of groundwater geochemistry data. Chemometrics and Intelligent Laboratory Systems, 60, 265–281.
Fisher, R. A. (1915). Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika, 10, 507–521.
Fisher, R. A. (1921). On the “probable error” of a coefficient of correlation deduced from a small sample. Metron, 1, 3–32.
Fitzgerald, W. F. (1999). Clean hands, dirty hands: Clair Patterson and the aquatic biogeochemistry of mercury. In C. I. Davidson (Ed.), Clean hands: Clair Patterson Crusade against environmental lead contamination (pp. 119–137). Commack: Nova Science Publishers.
Francis, S. S., Selvin, S., Yang, W., Buffler, P. A., & Wiemels, J. L. (2012). Unusual space-time patterning of the Fallon, Nevada leukemia cluster: Evidence of an infectious etiology. Chemico-Biological Interactions, 196, 102–109.
Gao, Y., & Mucci, A. (2003). Individual and competitive adsorption of phosphate and arsenate on goethite in artificial seawater. Chemical Geology, 199, 91–109.
Greenacre, M. J. (1984). Theory and application of correspondence analysis. London: Academic. 364 pp.
Gross, J. (2012). nortest: Tests for normality. R package version 1.0-1.
Gustafsson, J. P. (2003). Modelling molybdate and tungstate adsorption to ferrihydrite. Chemical Geology, 200, 105–115.
Gustafsson, J. P., & Bhattacharya, P. (2007). Geochemical modelling of arsenic adsorption onto oxide surfaces. In P. Bhattacharya, A. B. Mukherjee, J. Bundschuh, R. Zevenhoven, & R. H. Loeppert (Eds.), Arsenic in soil and groundwater environment: biogeochemical interactions, health effects and remediation. Trace metals and other contaminants in the environment (Vol. 9, pp. 159–206). Amsterdam: Elsevier.
Haque, S., & Johannesson, K. H. (2006). Arsenic concentrations and speciation along a groundwater flow path: The Carrizo sand aquifer, Texas, USA. Chemical Geology, 228, 57–71.
Haque, S., Ji, J., & Johannesson, K. H. (2008). Evaluating mobilization and transport of arsenic in sediments of Aquia aquifer, Maryland, USA. Journal of Contaminant Hydrology, 99, 68–84.
Harita, Y., Hori, T., & Sugiyama, M. (2005). Release of trace oxyanions from littoral sediments and suspended particles induced by pH increase in the epilimnion of lakes. Limnology and Oceanography, 50, 636–645.
Harvey, C. F., Swartz, C. H., Baduzzaman, A. B. M., Keon-Blute, N., Yu, W., Ali, M. A., et al. (2002). Arsenic mobility and groundwater extraction in Bangladesh. Science, 298, 1602–1606.
Herbelin, A. L., & Westall, J. C. (1999). FITEQL 4.0: A computer program for determination of chemical equilibrium constants from experimental data (Department of Chemistry Report 99–01). Corvallis: Oregon State University.
Islam, F. S., Gault, A. G., Boothman, C., Polya, D. A., Charnock, J. M., Chatterjee, D., et al. (2004). Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature, 430, 68–71.
Johannesson, K. H., & Neumann, K. (2013). Geochemical cycling of mercury in a deep, confined aquifer: Insights from biogeochemical reactive transport modeling. Geochimica et Cosmochimica Acta, 106, 25–43.
Johannesson, K. H., & Tang, J. (2009). Conservative behavior of arsenic and other oxyanion-forming trace elements in an oxic groundwater flow system. Journal of Hydrology, 378, 13–28.
Johannesson, K. H., Stetzenbach, K. J., Kreamer, D. K., & Hodge, V. F. (1996). Multivariate statistical analysis of arsenic and selenium in groundwaters from south–central Nevada and Death Valley, California. Journal of Hydrology, 178, 181–204.
Johannesson, K. H., Lyons, W. B., Huey, S., Doyle, G. A., Swanson, E. E., & Hackett, E. (1997). Oxyanion concentrations in eastern Sierra Nevada rivers: 2. Arsenic and phosphate. Aquatic Geochemistry, 3, 61–97.
Johannesson, K. H., Lyons, W. B., Graham, E. Y., & Welch, K. A. (2000). Oxyanion concentrations in eastern Sierra Nevada rivers: 3. Boron, molybdenum, vanadium, and tungsten. Aquatic Geochemistry, 6, 19–46.
Johannesson, K. H., Tang, J., Daniels, J. M., Bounds, W. J., & Burdige, D. J. (2004). Rare earth element concentrations and speciation in organic-rich blackwaters of the Great Dismal Swamp, Virginia, USA. Chemical Geology, 209, 271–294.
Johannesson, K. H., Dave, H. B., Mohajerin, T. J., & Datta, S. (2013). Controls on tungsten concentrations in groundwater flow systems: The role of adsorption, aquifer sediment Fe(III) oxide/oxyhydroxide content, and thiotungstate formation. Chemical Geology, 351, 76–94.
Kalinich, J. F., Emond, C. A., Dalton, T. K., Mog, S. R., Coleman, G. D., Kordell, J. E., et al. (2005). Embedded weapons-grade tungsteon alloy shrapnel rapidly induces metastatic high-grade rhabdomyosarcomas in F344 rats. Environmental Health Perspectives, 113, 729–734.
Kashiwabara, T., Takahashi, Y., Marcus, M. A., Uruga, T., Tanida, H., Terada, Y., et al. (2013). Tungsten species in natural ferromanganese oxides related to its different behavior from molybdenum in oxic ocean. Geochimica et Cosmochimica Acta, 106, 364–378.
Kelly, A. D. E., Lemaire, M., Young, Y. K., Eustache, J. H., Guilbert, C., Molina, M. F., et al. (2013). In vivo tungsten exposure alters B-cell development and increases DNA damage in murine bone marrow. Toxicological Sciences, 131, 434–446.
Kletzin, A., & Adams, M. W. W. (1996). Tungsten in biological systems. FEMS Microbiology Ecology, 18, 5–63.
Koutsospyros, A., Braida, W., Christodoulatos, C., Dermatas, D., & Strigul, N. (2006). A review of tungsten: Frrom environmental obscurity to scrutiny. Journal of Hazardous Materials, 136, 1–19.
Kowalski, C. J. (1972). On the effects of non-normality on the distribution of the sample product–moment correlation coefficient. Journal of the Royal Statistical Society: Series C: Applied Statistics, 21, 1–12.
Leybourne, M. I., Peter, J. M., Johannesson, K. H., & Boyle, D. R. (2008). The Lake St. Martin bolide has a big impact on groundwater fluoride concentrations. Geology, 36, 115–118.
Manning, B. A., & Goldberg, S. (1996). Modeling arsenate competitive adsorption on kaolinite, montmorillonite, and illite. Clays and Clay Minerals, 44, 609–623.
Marquet, P., François, B., Vignon, P., & Lachâtre, G. (1996). A soldier who had seizures after drinking quarter of a litre of wine. Lancet, 348, 1070.
McArthur, J. M., Ravenscroft, P., Banerjee, D. M., Milsom, J., Hudson-Edwards, K. A., Sengupta, S., et al. (2008). How paleosols influence groundwater flow and arsenic pollution: A model from the Bengal Basin and its worldwide implications. Water Resources Research, 44, W11411. doi:10.1029/2007/WR006552.
McCleskey, R. B., Nordstrom, D. K., & Maest, A. S. (2004). Preservation of water samples for arsenic(III/V) determinations: An evaluation of the literature and new analytical results. Applied Geochemistry, 19, 995–1009.
Métral, J., Charlet, L., Bureau, S., Basu Mallik, S., Charkraborty, S., Ahmed, K. M., et al. (2008). Comparison of dissolved and particulate arsenic distributions in shallow aquifers of Chakdaha, India, and Araihazar, Bangladesh. Geochemical Transactions, 9, 1. doi:10.1186/1467-4866-9-1.
Miyauchi, T., Iwashita, M., & Shimamura, T. (1998). Behaviors of trace elements in Tsukui Reservoir inferred from ICP-MS analysis. Journal of Environmental Chemistry, 8, 12–21.
Mukherjee, A., Fryar, A. E., & Thomas, W. A. (2009a). Geologic, geomorphic and hydrologic framework and evolution of the Bengal basin, India and Bangladesh. Journal of Asian Earth Sciences, 34, 227–244.
Mukherjee, A., Fryar, A. E., & O’Shea, B. M. (2009b). Major occurrences of elevated arsenic in groundwater and other natural waters. In K. Henke (Ed.), Arsenic: Environmental chemistry, health threats and waste treatment (pp. 303–350). Chichester: Wiley.
Mukherjee, A., Bhattacharya, P., Shi, F., Fryar, A. E., Mukherjee, A. B., Xie, Z. M., et al. (2009c). Chemical evolution in the high arsenic groundwater of the Huhhot basin (Inner Mongolia, PR China) and its difference from the western Bengal basin (India). Applied Geochemistry, 24, 1835–1851.
Neal, A. W. (2010). Hydrogeochemical and mineralogical evaluation of groundwater arsenic contamination in Murshidabad District, West Bengal, India (M.S. thesis, 128 pp.). Manhattan, KS: Kansas State Univ.
Nickson, R. T., McArthur, J. M., Ravenscroft, P., Burgess, W. G., & Ahmed, K. M. (2000). Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Applied Geochemistry, 15, 403–413.
Oleson, S. G., & Carr, J. R. (1990). Correspondence analysis of water quality data: implications for fauna deaths at Stillwater Lakes, Nevada. Mathematics Geology, 22, 665–698.
Oremland, R. S., & Stolz, J. F. (2005). Arsenic, microbes and contaminated aquifers. Trends in Microbiology, 13, 45–49.
Peão, M. N. D., Águas, A. P., Sá, C. M., & Grande, N. R. (1993). Inflammatory response of the lung to tungsten particles: an experimental study in mice submitted to intratracheal instillation of a calcium tungstate powder. Lung, 171, 187–201.
Ravenscroft, P., Brammer, H., Richards, K. (2009). Arsenic pollution: A global synthesis (588 pp).
Sahle, W., Krantz, S., Christensson, B., & Laszlo, I. (1996). Preliminary data on hard metal workers exposure to tungsten oxide fibres. Science of the Total Environment, 191, 153–167.
Seiler, R. L. (2012). Physical setting and natural sources of exposure to carcinogenic trace elements and radionuclides in Lahontan Valley, Nevada. Chemico-Biological Interactions, 196, 79–86.
Seiler, R. L., Stollenwerk, K. G., & Garbarino, J. R. (2005). Factors controlling tungsten concentrations in ground water, Carson Desert, Nevada. Applied Geochemistry, 20, 423–441.
Sheppard, P. R., Ridenour, G., Speakman, R. J., & Witten, M. L. (2006). Elevated tungsten and cobalt in airborne particulates in Fallon, Nevada: Possible implications for the childhood leukemia cluster. Applied Geochemistry, 21, 152–165.
Sheppard, P. R., Speakman, R. J., Ridenour, G., & Witten, M. L. (2007). Temporal variability of tungsten and cobalt in Fallon, Nevada. Environmental Health Perspectives, 115, 715–719.
Smith, R. M., Martell, A. E. (2004). NIST critically selected stability constants of metal complexes database. NIST Standard References Database 46, Version 8.0.
Sprince, N. L., Oliver, L. C., Chamberlain, R. I., Eisen, E. A., & Greene, R. E. (1994). Etiology and pathogenesis of hard metal disease. Science of the Total Environment, 150, 55.
Strigul, N. (2010). Does speciation matter for tungsten ecotoxicology? Ecotoxicology and Environmental Safety, 73, 1099–1113.
Sur, P. (2006). Mineralogical and geochemical investigations of sediments and waters from river Ganges with special application to arsenic contamination of groundwaters in the aquifers of Murshidabad district, West Bengal (Masters thesis, pp. 103). University of Calcutta.
Thomas, J. M., Benedict, F. C., Jr., Rose, T. P., Hershey, R. L., Paces, J. B., Peterman, Z. E., et al. (2002). Geochemical and isotopic interpretations of groundwater flow in the Oasis Valley flow system. Southern Nevada: Desert Research Institute. Publication No. 40190.
Tummers, B. (2006). DataThief III. http://datathief.org. Accessed 7 February 2013.
U.S. EPA. (2008). Emerging contaminant–tungsten. Fact Sheet. EPA 505-F-07-005.
van Geen, A., Rose, J., Thoral, S., Garnier, J. M., Zheng, Y., & Bottero, J. Y. (2004). Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions: Part II. Evidence from sediment incubations. Geochimica et Cosmochimica Acta, 68, 3475–3486.
Venables, W. N., & Ripley, B. D. (2002). Modern applied statistics with S (4th ed.). New York: Springer. 0-387-95457-0.
Welch, A. H., & Lico, M. S. (1998). Factors controlling As and U in shallow ground water, southern Carson Desert, Nevada. Applied Geochemistry, 13, 521–539.
White, A. F., & Chuma, N. J. (1987). Carbon and isotopic mass balance models of Oasis Valley–Fortymile Canyon groundwater basin, southern Nevada. Water Resources Research, 23, 571–582.
Wilkie, J. A., & Hering, J. G. (1998). Rapid oxidation of geothermal arsenic(III) in streamwaters of the eastern Sierra Nevada. Environmental Science & Technology, 32, 657–662.
Zheng, Y., Stute, M., van Geen, A., Gavrieli, I., Dhar, R., Simpson, H. J., et al. (2004). Redox control of arsenic mobilization in Bangladesh groundwater. Applied Geochemistry, 19, 201–214.
Acknowledgments
This work was supported by NSF awards EAR-1014946 to Johannesson and EAR-1014971 to Datta. We thank the editor J. T. Trevors and the anonymous reviewers whose comments greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohajerin, T.J., Neal, A.W., Telfeyan, K. et al. Geochemistry of Tungsten and Arsenic in Aquifer Systems: A Comparative Study of Groundwaters from West Bengal, India, and Nevada, USA. Water Air Soil Pollut 225, 1792 (2014). https://doi.org/10.1007/s11270-013-1792-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1792-x