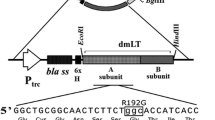
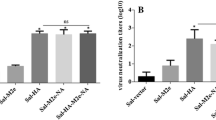
Abstract
The eukaryotic expression plasmid of M2e-Hsp70 is a candidate M2e-based DNA vaccine. In order to evaluate the immunization potential of this construct, Specific Pathogen Free chickens were immunized either intramuscularly or orally. Mutant Salmonella typhimurium was used as carrier for oral delivery of the M2e-Hsp70 construct. M2e-specific humoral and cellular responses were tested by ELISA and Lymphocyte Proliferation Assay, respectively. Our results indicate that both humoral and cellular immune responses are conferred against M2e-Hsp70 plasmid in either of the intramuscular or oral routes of administration; however, these responses are significantly higher in intramuscular injection in contrast to oral administration. When it comes to mass vaccination of commercial chicken flocks oral administration is preferred due to the ease of application as well as its capability of eliciting mucosal, humoral and cellular immune responses; so measurements should be taken to improve the immunization potency of our orally delivered DNA vaccine.


Similar content being viewed by others
References
Ameiss K, Ashraf S, Kong W, Pekosz A, Wu WH, Milich D, Billaud JN, Curtiss R (2010) Delivery of woodchuck hepatitis virus-like particle presented influenza M2e by recombinant attenuated Salmonella displaying a delayed lysis phenotype. Vaccine 28:6704–6713
Ashby D, Leduc I, Lauzon W, Lee BC, Singhal N, Cameron DW (2005) Attenuated Salmonella typhimurium SL3261 as a vaccine vector for recombinant antigen in rabbits. J Immunol Methods 299:153–164
Ashraf S, Kong W, Wang SF, Yang JS, Curtiss R (2011) Protective cellular responses elicited by vaccination with influenza nucleoprotein delivered by a live recombinant attenuated Salmonella vaccine. Vaccine 29:3990–4002
Babapoor S, Neef T, Mittelholzer C, Girshick T, Garmendia A, Shang H, Khan MI, Burkhard P (2011) A Novel Vaccine Using Nanoparticle Platform to Present Immunogenic M2e against Avian Influenza Infection. Influenza research and treatment 2011: Article ID 126794
Darji A, Zur Lage S, Garbe AI, Chakraborty T, Weiss S (2000) Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol Med Microbiol 27:341–349
Denis J, Acosta-Ramirez E, Zhao Y, Hamelin ME, Koukavica I, Baz M, Abed Y, Savard C, Pare C, Lopez Macias C, Boivin G, Leclerc D (2008) Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine 26:3395–3403
Donnelly JJ, Wahren B, Liu MA (2005) DNA vaccines: Progress and challenges. J Immunol 175:633–639
Fagan PK, Walker MJ, Chin J, Eamens GJ, Djordjevic SP (2001) Oral immunization of swine with attenuated Salmonella typhimurium aroA SL3261 expressing a recombinant antigen of Mycoplasma hyopneumoniae (NrdF) primes the immune system for a NrdF specific secretory IgA response in the lungs. Microb Pathog 30:101–110
Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, Schotsaert M, Birkett A, Saelens X (2009) M2e-based universal influenza A vaccine. Vaccine 27:6280–6283
Fioretti D, Iurescia S, Fazio VM, Rinaldi M (2010) DNA Vaccines: Developing New Strategies against Cancer. J Biomed Biotechnol 2010: Article ID 174378
Frace AM, Klimov AI, Rowe T, Black RA, Katz JM (1999) Modified M2 proteins produce heterotypic immunity against influenza A virus. Vaccine 17:2237–2244
Gosling JP (2000) Immunoassays: A Practical Approach. Oxford University Press, London
Guo Z, Wang H, Yang T, Wang X, Lu D, Li Y, Zhang Y (2010) Priming with a DNA vaccine and boosting with an inactivated vaccine enhance the immune response against infectious bronchitis virus. J Virol Methods 167:84–89
Haygreen L, Davison F, Kaiser P (2005) DNA vaccines for poultry: the jump from theory to practice. Expert Rev Vaccines 4:51–62
Hoiseth SK, Stocker BAD (1981) Aromatic-Dependent Salmonella-Typhimurium Are Non-Virulent and Effective as Live Vaccines. Nature 291:238–239
Huang J, Xie Y, Zhang Q, Shen X, Ren D (2000) Immune responses of a recombined live Salmonella typhimurium SL3261 expressing a multi-epitope antigen of HCV. Acta Microbiol Sin 40:495–499
Ionescu RM, Przysiecki CT, Liang X, Garsky VM, Fan J, Wang B, Troutman R, Rippeon Y, Flanagan E, Shiver J, Shi L (2006) Pharmaceutical and immunological evaluation of human papillomavirus viruslike particle as an antigen carrier. J Pharm Sci 95:70–79
Jazi MH, Dabaghian M, Tebianian M, Gharagozlou MJ, Ebrahimi SM (2012) In vivo electroporation enhances immunogenicity and protection against influenza A virus challenge of an M2e-HSP70c DNA vaccine. Virus Res 167:219–255
Jiao ZY, Chen MH, Zhu SL, Li LG, Chen J, Hu PJ (2003) Establishment of an attenuated Salmonella typhimurium vaccine strain SL3261 expressing Hp-NAP gene. CHINESE J Cell Mol Immunol 19:335–337
Jiao H, Pan Z, Yin Y, Geng S, Sun L, Jiao X (2011) Oral and nasal DNA vaccines delivered by attenuated Salmonella enterica serovar Typhimurium induce a protective immune response against infectious bronchitis in chickens. Clin Vaccine Immunol 18:1041–1045
Kong W, Wanda SY, Zhang X, Bollen W, Tinge SA, Roland KL, Curtiss R (2008) Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc Natl Acad Sci U S A 105:9361–9366
Kong W, Brovold M, Koeneman BA, Clark-Curtiss J, Curtiss R (2012) Turning self-destructing Salmonella into a universal DNA vaccine delivery platform. Proc Natl Acad Sci U S A 109:19414–19419
Layton SL, Kapczynski DR, Higgins S, Higgins J, Wolfenden AD, Liljebjelke KA, Bottje WG, Swayne D, Berghman LR, Kwon YM, Hargis BM, Cole K (2009) Vaccination of chickens with recombinant Salmonella expressing M2e and CD154 epitopes increases protection and decreases viral shedding after low pathogenic avian influenza challenge. Poult Sci 88:2244–2252
Liu MA (2003) DNA vaccines: a review. J Intern Med 253:402–410
Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W (1999) A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med 5:1157–1163
Ning JF, Zhu W, Xu JP, Zheng CY, Meng XL (2009) Oral delivery of DNA vaccine encoding VP28 against white spot syndrome virus in crayfish by attenuated Salmonella typhimurium. Vaccine 27:1127–1135
Paglia P, Medina E, Arioli I, Guzman CA, Colombo MP (1998) Gene transfer in dendritic cells, induced by oral DNA vaccination with Salmonella typhimurium, results in protective immunity against a murine fibrosarcoma. Blood 92:3172–3176
Pan Z, Zhang X, Geng S, Cheng N, Sun L, Liu B, Huang J, Jiao X (2009) Priming with a DNA vaccine delivered by attenuated Salmonella typhimurium and boosting with a killed vaccine confers protection of chickens against infection with the H9 subtype of avian influenza virus. Vaccine 27:1018–1023
Pan Z, Zhang X, Geng S, Fang Q, You M, Zhang L, Jiao X, Liu X (2010) Prime-boost immunization using a DNA vaccine delivered by attenuated Salmonella enterica serovar Typhimurium and a killed vaccine completely protects chickens from H5N1 highly pathogenic avian influenza virus. Clin Vaccine Immunol 17:518–523
Slepushkin VA, Katz JM, Black RA, Gamble WC, Rota PA, Cox NJ (1995) Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine 13:1399–1402
Trampuz A, Prabhu RM, Smith TF, Baddour LM (2004) Avian influenza: a new pandemic threat? Mayo Clin Proc 79:523–530
Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, Kavita U, Stanberry L, Shaw A (2011) Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 29:5145–5152
Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T (2002) Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol 169:2422–2429
Wang BZ, Gill HS, Kang SM, Wang L, Wang YC, Vassilieva EV, Compans RW (2012) Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clin Vaccine Immunol 19:1119–1125
Wu F, Huang JH, Yuan XY, Huang WS, Chen YH (2007) Characterization of immunity induced by M2e of influenza virus. Vaccine 25:8868–8873
Wu F, Yuan XY, Huang WS, Chen YH (2009a) Heterosubtypic protection conferred by combined vaccination with M2e peptide and split influenza vaccine. Vaccine 27:6095–6101
Wu F, Yuan XY, Li J, Chen YH (2009b) The co-administration of CpG-ODN influenced protective activity of influenza M2e vaccine. Vaccine 27:4320–4324
Zhao G, Du L, Xiao W, Sun S, Lin Y, Chen M, Kou Z, He Y, Lustigman S, Jiang S, Zheng BJ, Zhou Y (2010) Induction of protection against divergent H5N1 influenza viruses using a recombinant fusion protein linking influenza M2e to Onchocerca volvulus activation associated protein-1 (ASP-1) adjuvant. Vaccine 28:7233–7240
Acknowledgments
This project was financially supported by grant no. 7502015/6/16 supplied by University of Tehran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assadian, F., Nikbakht, G., Niazi, S. et al. Immune responses to oral and IM administration of M2e-Hsp70 construct. Vet Res Commun 38, 157–163 (2014). https://doi.org/10.1007/s11259-014-9599-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-014-9599-9