Abstract
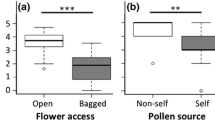
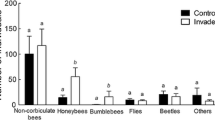
The York gum–jam woodlands of southwest Western Australia support diverse annual wildflower communities despite extensive habitat fragmentation, remnant isolation and the invasion of many exotic annual plant species. Few studies have explored the pollinator–plant relationships maintaining these persistently species-rich ‘novel’ communities. We examine the pollination ecology of five native species common to York gum–jam woodland annual communities to determine whether native pollinators may be mediating impacts of exotic annual plants on native wildflower species. We determined the pollination requirements of native focal species and the diversity and frequency of pollinator visitation to these focal plant species across invasion gradients. We also recorded the pollinator community of a dominant exotic herb in this system: Arctotheca calendula (cape weed). Only two of the five native species examined had significant seed set benefits attributable to insect pollination. One native plant species, Podotheca gnaphalioides, had pollinator assemblages that overlapped significantly with exotic A. calendula, with some reduction in pollinator visitation evident. One species, Waitzia acuminata, was found to benefit from insect pollination only in the larger of two surveyed remnants, which may reflect emerging reproductive polymorphism among geographically isolated populations. We highlight two mechanisms in this system that may buffer pollinator-mediated impacts of exotic species on native species: autonomous seed production, which may be increasingly prevalent in isolated populations, and segregation of pollinator resources among species. Our findings illustrate the ways that pollinator-mediated interactions can affect seed set within plant communities persisting in highly fragmented and invaded agricultural landscapes.




Similar content being viewed by others
References
Abdala-Roberts L, Marrufo-Zapata D, Arceo-Gómez G, Parra-Tabla V (2014) Pollen limitation, fruit abortion, and autonomous selfing in three populations of the perennial herb Ruellia nudiflora. Plant Species Biol 29:25–33. doi:10.1111/j.1442-1984.2012.00392.x
Anderson DR, Burnham KP, Thompson WL (2000) Null hypothesis testing: problems, prevalence, and an alternative. J Wildl Manag 64:912–923. doi:10.2307/3803199
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: Guide to software and statistical methods. PRIMER-E, Plymouth
Bartomeus I, Bosch J, Vilà M (2008a) High invasive pollen transfer, yet low deposition on native stigmas in a Carpobrotus-invaded community. Ann Bot 102:417–424
Bartomeus I, Vilà M, Santamaría L (2008b) Contrasting effects of invasive plants in plant–pollinator networks. Oecologia 155:761–770. doi:10.1007/s00442-007-0946-1
Bates D, Maechler M (2009) R package: lme4, linear mixed-effects models using S4 classes
Beard JS (1990) Plant life in Western Australia. Kangaroo Press, Kenthurst
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. doi:10.1016/j.tree.2008.10.008
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Carvalheiro LG, Biesmeijer JC, Benadi G et al (2014) The potential for indirect effects between co-flowering plants via shared pollinators depends on resource abundance, accessibility and relatedness. Ecol Lett 17:1389–1399. doi:10.1111/ele.12342
Dietzsch A, Stanley D, Stout J (2011) Relative abundance of an invasive alien plant affects native pollination processes. Oecologia 167:469–479. doi:10.1007/s00442-011-1987-z
Dwyer JM, Hobbs RJ, Wainwright CE, Mayfield MM (2015) Climate moderates release from nutrient limitation in natural annual plant communities. Global Ecolog Biogeogr 24(5):549–561. doi:10.1111/geb.12277
Eaton DAR, Fenster CB, Hereford J, Huang S-Q, Ree RH (2012) Floral diversity and community structure in Pedicularis (Orobanchaceae). Ecology 93:S182–S194. doi:10.1890/11-0501.1
Feldman TS, Morris WF, Wilson WG (2004) When can two plant species facilitate each other’s pollination? Oikos 105:197–207. doi:10.2307/3547899
Fiedler AK, Landis DA, Arduser M (2012) Rapid shift in pollinator communities following invasive species removal. Restor Ecol 20:593–602. doi:10.1111/j.1526-100X.2011.00820.x
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304. doi:10.2307/3599633
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474. doi:10.1016/j.tree.2004.07.005
Hester AJ, Hobbs RJ (1992) Influence of fire and soil nutrients on native and non-native annuals at remnant vegetation edges in the Western Australian wheatbelt. J Veg Sci 3:101–108. doi:10.2307/3236003
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. doi:10.1002/bimj.200810425
Hurvich CM, Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76:297–307. doi:10.1093/biomet/76.2.297
Jakobsson A, Lázaro A, Totland Ø (2009) Relationships between the floral neighborhood and individual pollen limitation in two self-incompatible herbs. Oecologia 160:707–719. doi:10.2307/40310073
Joar Hegland S, Totland Ø (2008) Is the magnitude of pollen limitation in a plant community affected by pollinator visitation and plant species specialisation levels? Oikos 117:883–891. doi:10.1111/j.0030-1299.2008.16561.x
Kaiser-Bunbury CN, Valentin T, Mougal J, Matatiken D, Ghazoul J (2011) The tolerance of island plant–pollinator networks to alien plants. J Ecol 99:202–213. doi:10.1111/j.1365-2745.2010.01732.x
Kalisz S, Vogler DW, Hanley KM (2004) Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature 430:884–887
Karron JD, Thumser NN, Tucker R, Hessenauer AJ (1995) The influence of population density on outcrossing rates in Mimulus ringens. Heredity 75:175–180
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Niwot
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Syst 29:83–112. doi:10.2307/221703
Kelly D, Ladley JJ, Robertson AW (2007) Is the pollen-limited mistletoe Peraxilla tetrapetala (Loranthaceae) also seed limited? Aust Ecol 32:850–857
Kirchner F et al (2005) Effects of local density on insect visitation and fertilization success in the narrow-endemic Centaurea corymbosa (Asteraceae). Oikos 111:130–142. doi:10.1111/j.0030-1299.2005.14022.x
Knight TM et al (2005) Pollen Limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497. doi:10.2307/30033813
Lai HR, Mayfield MM, Gay-des-Combes J, Spiegelberger T, Dwyer JM (2015) Distinct invasion strategies operating within a natural annual plant system. Ecol Lett 18:336–346
Law W, Salick J, Knight T (2010) The effects of pollen limitation on population dynamics of snow lotus (Saussurea medusa and S. laniceps, Asteraceae): threatened Tibetan medicinal plants of the eastern Himalayas. Plant Ecol 210:343–357. doi:10.1007/s11258-010-9761-6
Lázaro A, Lundgren R, Totland Ø (2014) Experimental reduction of pollinator visitation modifies plant–plant interactions for pollination. Oikos 123:1037–1048. doi:10.1111/oik.01268
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Review paper. Mechanisms underlying the impacts of exotic plant invasions. Proc Biol Sci 270:775–781. doi:10.2307/3558605
Moeller DA, Geber MA (2005) Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution 59:786–799. doi:10.2307/3449026
Olito C, Fox JW (2015) Species traits and abundances predict metrics of plant–pollinator network structure, but not pairwise interactions. Oikos 124:428–436. doi:10.1111/oik.01439
Pauw A, Bond WJ (2011) Mutualisms matter: pollination rate limits the distribution of oil-secreting orchids. Oikos 120:1531–1538. doi:10.1111/j.1600-0706.2011.19417.x
Pleasants JM (1980) Competition for bumblebee pollinators in Rocky Mountain plant communities. Ecology 61:1446–1459. doi:10.2307/1939053
Primer-E Ltd (2008) Primer 6, version 6.1.11 + PERMANOVA version 1.0.1. Primer-E Ltd., Plymouth
R Development Core Team (2012) R: A language and environment for statistical computing. R foundation for Statistical Computing, Vienna
Raine N, Pierson A, Stone G (2007) Plant–pollinator interactions in a Mexican Acacia community. Arthropod-Plant Interactions 1:101–117. doi:10.1007/s11829-007-9010-7
Rosenheim JA, Williams NM, Schreiber SJ (2014) Parental optimism versus parental pessimism in plants: how common should we expect pollen limitation to be? Am Nat 184:75–90. doi:10.1086/676503
Steenhuisen S-L, Johnson SD (2012) Evidence for autonomous selfing in grassland Protea species (Proteaceae). Bot J Linn Soc 169:433–446. doi:10.1111/j.1095-8339.2012.01243.x
Steffan-Dewenter I, Munzenberg U, Tscharntke T (2001) Pollination, seed set and seed predation on a landscape scale. Proc R Soc Lond 268:1685–1690. doi:10.1098/rspb.2001.1737
Suter M (2009) Reproductive allocation of Carex flava reacts differently to competition and resources in a designed plant mixture of five species. Plant Ecol 201:481–489. doi:10.1007/s11258-008-9499-6
Wagenmakers E-J, Farrell S (2004) AIC model selection using Akaike weights. Psychon Bull Rev 11:192–196. doi:10.3758/BF03206482
Wang P, Weiner J, Cahill JF, Zhou DW, Bian HF, Song YT, Sheng LX (2014) Shoot competition, root competition and reproductive allocation in Chenopodium acuminatum. J Ecol 102:1688–1696. doi:10.1111/1365-2745.12313
Waters SM, Fisher SE, Hille Ris Lambers J (2014) Neighborhood-contingent indirect interactions between native and exotic plants: multiple shared pollinators mediate reproductive success during invasions. Oikos 123:433–440. doi:10.1111/j.1600-0706.2013.00643.x
White EM, Wilson JC, Clarke AR (2006) Biotic indirect effects: a neglected concept in invasion biology. Divers Distrib 12:443–455. doi:10.1111/j.1366-9516.2006.00265.x
Wilcove DS, Rothstein D, Jason D, Phillips A, Losos E (1998) Quantifying threats to imperiled species in the United States. Bioscience 48:607–615. doi:10.2307/1313420
Acknowledgments
This work was made possible by grants to MM Mayfield by the ARC (DP1094413 and DP140100574). We would like to thank the ERIE group at the University of Western Australia for logistical support. We would also like to thank John Dwyer, Michael Sams, Hao Ran Lai and Tobias Smith for consultation on statistical analyses, and Tobias Smith for help with insect identifications.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Philip Ladd.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Loy, X., Wainwright, C.E. & Mayfield, M.M. Asteraceae invaders have limited impacts on the pollination of common native annual species in SW Western Australia’s open woodland wildflower communities. Plant Ecol 216, 1103–1115 (2015). https://doi.org/10.1007/s11258-015-0495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-015-0495-3