Abstract
Purpose
Considering the referred beneficial effects of protein restriction on diabetic nephropathy (DN) and the role of renal endothelium in its pathogenesis, we evaluated the effect of general control nonderepressible 2 (GCN2) kinase activation, a sensor of amino acid deprivation, on known detrimental molecular pathways in primary human glomerular endothelial cells (GEnC).
Methods
GEnC were cultured under normal or high-glucose conditions in the presence or not of the GCN2 kinase activator, tryptophanol. Glucose transporter 1 (GLUT1) expression was assessed by western blotting and reactive oxygen species (ROS) using a fluorogenic probe. Activities of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and protein kinase C (PKC) were assessed by commercial activity assays, sorbitol colorimetrically, methylglyoxal by ELISA and O-linked β-N-acetyl glucosamine (O-GlcNAc)-modified proteins by western blotting.
Results
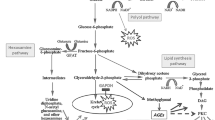
High glucose induced GLUT1 expression, increased ROS and inhibited GAPDH. Also it increased the polyol pathway product sorbitol, PKC activity, the level of the O-GlcNAc-modified proteins that produced by the hexosamine pathway and the advanced glycation endproducts’ precursor methylglyoxal. Co-treatment of GEnC with tryptophanol restored the above high-glucose-induced alterations.
Conclusions
Activation of GCN2 kinase protects GEnC from high-glucose-induced harmful molecular pathways. By inhibiting concurrently many pathways involved in DN pathogenesis, GCN2 kinase may serve as a pharmaceutical target for the treatment of DN.




Similar content being viewed by others
References
Forbes JM, Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93(1):137–188. doi:10.1152/physrev.00045.2011
Sena CM, Pereira AM, Seica R (2013) Endothelial dysfunction—a major mediator of diabetic vascular disease. Biochim Biophys Acta 1832(12):2216–2231. doi:10.1016/j.bbadis.2013.08.006
Burrows NR, Li Y, Geiss LS (2010) Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 33(1):73–77. doi:10.2337/dc09-0343
Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, Investigators HS (2001) Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286(4):421–426
Eleftheriadis T, Antoniadi G, Pissas G, Liakopoulos V, Stefanidis I (2013) The renal endothelium in diabetic nephropathy. Ren Fail 35(4):592–599. doi:10.3109/0886022X.2013.773836
Brosius FC, Heilig CW (2005) Glucose transporters in diabetic nephropathy. Pediatr Nephrol 20(4):447–451. doi:10.1007/s00467-004-1748-x
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Castilho BA, Shanmugam R, Silva RC, Ramesh R, Himme BM, Sattlegger E (2014) Keeping the eIF2 alpha kinase Gcn2 in check. Biochim Biophys Acta 1843(9):1948–1968. doi:10.1016/j.bbamcr.2014.04.006
Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD (2014) FGF21 is an endocrine signal of protein restriction. J Clin Investig 124(9):3913–3922. doi:10.1172/JCI74915
Walker JD, Bending JJ, Dodds RA, Mattock MB, Murrells TJ, Keen H, Viberti GC (1989) Restriction of dietary protein and progression of renal failure in diabetic nephropathy. Lancet 2(8677):1411–1415
Zeller K, Whittaker E, Sullivan L, Raskin P, Jacobson HR (1991) Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med 324(2):78–84. doi:10.1056/NEJM199101103240202
Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Tsogka K, Sounidaki M, Stefanidis I (2016) Differential effects of the two amino acid sensing systems, the GCN2 kinase and the mTOR complex 1, on primary human alloreactive CD4 + T-cells. Int J Mol Med 37(5):1412–1420. doi:10.3892/ijmm.2016.2547
Eleftheriadis T, Pissas G, Antoniadi G, Spanoulis A, Liakopoulos V, Stefanidis I (2014) Indoleamine 2,3-dioxygenase increases p53 levels in alloreactive human T cells, and both indoleamine 2,3-dioxygenase and p53 suppress glucose uptake, glycolysis and proliferation. Int Immunol 26(12):673–684. doi:10.1093/intimm/dxu077
Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC (2003) Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 23(16):5651–5663
Eleftheriadis T, Pissas G, Yiannaki E, Markala D, Arampatzis S, Antoniadi G, Liakopoulos V, Stefanidis I (2013) Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte reaction affects glucose influx and enzymes involved in aerobic glycolysis and glutaminolysis in alloreactive T-cells. Hum Immunol 74(12):1501–1509. doi:10.1016/j.humimm.2013.08.268
Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Investig 112(7):1049–1057. doi:10.1172/JCI18127
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404(6779):787–790. doi:10.1038/35008121
Gabbay KH, Merola LO, Field RA (1966) Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science 151(3707):209–210
Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL (1997) Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Investig 100(1):115–126. doi:10.1172/JCI119503
Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL (2000) Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation 101(6):676–681
Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R (2000) Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J Off Publ Fed Am Soc Exp Biol 14(3):439–447
Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED (1998) High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Investig 101(1):160–169. doi:10.1172/JCI119875
Wells L, Hart GW (2003) O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett 546(1):154–158
Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97(22):12222–12226. doi:10.1073/pnas.97.22.12222
Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Investig 108(9):1341–1348. doi:10.1172/JCI11235
Queisser MA, Yao D, Geisler S, Hammes HP, Lochnit G, Schleicher ED, Brownlee M, Preissner KT (2010) Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 59(3):670–678. doi:10.2337/db08-1565
Giacco F, Du X, D’Agati VD, Milne R, Sui G, Geoffrion M, Brownlee M (2014) Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 63(1):291–299. doi:10.2337/db13-0316
Brouwers O, Niessen PM, Miyata T, Ostergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, Sieber J, Mundel PH, Brownlee M, Janssen BJ, De Mey JG, Stehouwer CD, Schalkwijk CG (2014) Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 57(1):224–235. doi:10.1007/s00125-013-3088-5
Sato S, Kawamura H, Takemoto M, Maezawa Y, Fujimoto M, Shimoyama T, Koshizaka M, Tsurutani Y, Watanabe A, Ueda S, Halevi K, Saito Y, Yokote K (2009) Halofuginone prevents extracellular matrix deposition in diabetic nephropathy. Biochem Biophys Res Commun 379(2):411–416. doi:10.1016/j.bbrc.2008.12.088
Pines M, Spector I (2015) Halofuginone—the multifaceted molecule. Molecules 20(1):573–594. doi:10.3390/molecules20010573
Passariello N, Sepe J, Marrazzo G, De Cicco A, Peluso A, Pisano MC, Sgambato S, Tesauro P, D’Onofrio F (1993) Effect of aldose reductase inhibitor (tolrestat) on urinary albumin excretion rate and glomerular filtration rate in IDDM subjects with nephropathy. Diabetes Care 16(5):789–795
Cherney DZ, Konvalinka A, Zinman B, Diamandis EP, Soosaipillai A, Reich H, Lorraine J, Lai V, Scholey JW, Miller JA (2009) Effect of protein kinase Cbeta inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diabetes Care 32(1):91–93. doi:10.2337/dc08-1609
Funding
This study was funded by the resources of our department and by the Research Committee of the University of Thessaly (4962.01.28).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Eleftheriadis, T., Tsogka, K., Pissas, G. et al. Activation of general control nonderepressible 2 kinase protects human glomerular endothelial cells from harmful high-glucose-induced molecular pathways. Int Urol Nephrol 48, 1731–1739 (2016). https://doi.org/10.1007/s11255-016-1377-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1377-x