Abstract
Purpose
The diagnosis of Gleason score (GS) ≥7 with distinction from GS < 7 remains a difficult problem instructing clinical decisions. Moreover, the present wide application of prostate biopsy to increase prostate cancer detection rate might cause unnecessary and excessive examination or treatment. Therefore, a risk assessment model for forecasting GS ≥ 7 in potential prostate cancer patients was established to reduce unnecessary prostate biopsies.
Methods
Patients (n = 981; September 2009 to January 2013) who underwent trans-rectal ultrasound (TRUS)-guided core prostate biopsy were retrospectively evaluated in the first stage of the study. Age, prostate-specific antigen (PSA), free PSA (fPSA), the free/total PSA ratio (f/t), prostate volume (PV), PSA density (PSAD), digital rectal examination (DRE) findings (texture, nodules) and B-ultrasound detection results (normal or abnormal, presence of hypoechoic mass or microcalcification) were considered as potential predictive factors. After multiple logistic regression analysis, independent variables used to build a nomogram were selected using a backward elimination selection procedure. Then, a model to forecast GS ≥ 7 was designed for potential prostate cancer patients. In the second stage of the study, 410 cases (January 2013 to March 2015) were subsequently evaluated using our model for prostate biopsies, and the outcomes of biopsies were compared between the two stages.
Results
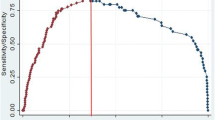
PSA, DRE texture, DRE nodules and B-ultrasound results were finally brought into our nomogram; a obviously greater area under the receiver operating characteristic (ROC) curve was obtained for the model than utilizing PSA, fPSA or PSAD alone (0.831 vs. 0.803, 0.770, 0.780 separately). We thereafter sought the best cutoff value in the ROC curve at 0.87, which provided sensitivity as high as 90 %. Meanwhile, the specificity was 45.8 %, which was much higher than the specificity of PSA, fPSA and PSAD at the same sensitivity level (37.7, 24.6 and 35.2 %, respectively). In the first stage, the detection rate of GS ≥ 7 in the high-risk group was significantly higher than in the low-risk group (80.3 vs. 35.0 %, p < 0.001). Furthermore, in the second stage, with the application of the new model associated with our former models, the rate of GS ≥ 7 was improved from 71.0 (697/981) to 79.2 % (267/337) (p = 0.003).
Conclusions
The model for forecasting GS ≥ 7 is effective, which could reduce unnecessary prostate biopsies without delaying patients’ diagnoses and treatments.

Similar content being viewed by others
References
Jemal A, Siegel R, Xu J et al (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Siegel R, Ma J, Zou Z et al (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Van Dong H, Lee AH, Nga NH et al (2014) Epidemiology and prevention of prostate cancer in Vietnam. Asian Pac J Cancer Prev 15:9747–9751
Matsuda T, Saika K (2009) Comparison of time trends in prostate cancer incidence (1973–2002) in Asia, from cancer incidence in five continents, Vols IV–IX. Jpn J Clin Oncol 39:468–469
Akbari ME, Hosseini SJ, Rezaee A et al (2008) Incidence of genitourinary cancers in the Islamic Republic of Iran: a survey in 2005. Asian Pac J Cancer Prev 9:549–552
Ploussard G, Epstein JI, Montironi R et al (2011) The contemporary concept of significant versus insignificant prostate cancer. Eur Urol 60:291–303
Gleason DF, Mellinger GT (1974) Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 111:58–64
Mellinger GT, Gleason D, Bailar J (1967) The histology and prognosis of prostatic cancer. J Urol 97:331–337
Epstein JI, Amin M, Boccon-Gibod L et al (2005) Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl 216:34–63
Dong F, Wang C, Farris AB et al (2012) Impact on the clinical outcome of prostate cancer by the 2005 international society of urological pathology modified Gleason grading system. Am J Surg Pathol 36:838–843
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65:467–479
Epstein JI, Allsbrook WC, Amin MB et al (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 29:1228–1242
Giunchi F, Brunocilla E, Borghesi M et al (2014) Revised Gleason grading system is a better predictor of indolent prostate cancer at the time of diagnosis: retrospective clinical-pathological study on matched biopsy and radical prostatectomy specimens. Clin Genitourin Cancer 12(5):325–329
Ukimura O, Coleman JA, de la Taille A et al (2013) Contemporary role of systematic prostate biopsies: indications, techniques, and implications for patient care. Eur Urol 63(2):214–230
Scattoni V, Maccagnano C, Zanni G et al (2010) Is extended and saturation biopsy necessary? Int J Urol 17(5):432–447
Wang X, Yu J, Sreekumar A et al (2005) Autoantibody signatures in prostate cancer. N Engl J Med 353:1224–1235
Schipper M, Wang G, Giles N et al (2015) Novel prostate cancer biomarkers derived from autoantibody signatures. Transl Oncol 8:106–111
Ma W, Diep K, Fritsche HA et al (2014) Diagnostic and prognostic scoring system for prostate cancer using urine and plasma biomarkers. Genet Test Mol Biomark 18:156–163
Vedder MM, de Bekker-Grob EW, Lilja HG et al (2014) The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men. Eur Urol 66:1109–1115
McDonald M, Parsons JK (2016) 4-Kallikrein test and kallikrein markers in prostate cancer screening. Urol Clin North Am 43:39–46
Dianat SS, Carter HB, Macura KJ et al (2014) Performance of multiparametric magnetic resonance imaging in the evaluation and management of clinically low-risk prostate cancer. Urol Oncol 32:39e1
Zhao R, Huang Y, Cheng G et al (2014) Developing a follow-up strategy for patients with PSA ranging from 4 to 10 ng/ml via a new model to reduce unnecessary prostate biopsies. PLoS One 9(9):e106933
Huang Y, Cheng G, Liu B et al (2014) A prostate biopsy strategy based on a new clinical nomogram reduces the number of biopsy cores required in high-risk patients. BMC Urol 11(14):8
Hodge KK, McNeal JE, Stamey TA (1989) Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol 142:66–70
Sherwin JC, Mirmilstein G, Pedersen J et al (2010) Tumor volume in radical prostatectomy specimens assessed by digital image analysis software correlates with other prognostic factors. J Urol 183:1808–1814
Thompson IM, Pauler DK, Goodman PJ et al (2004) Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 350:2239–2246
Catalona WJ, Richie JP, Ahmann FR et al (1994) Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol 151:1283–1290
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Funding
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Jiangsu Provincial Special Program of Medical Science (BL2012027).
Additional information
Xiao Li, Yongsheng Pan and Yuan Huang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, X., Pan, Y., Huang, Y. et al. Developing a model for forecasting Gleason score ≥7 in potential prostate cancer patients to reduce unnecessary prostate biopsies. Int Urol Nephrol 48, 535–540 (2016). https://doi.org/10.1007/s11255-016-1218-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1218-y