Abstract
Aims of study
It is reported that severe bladder disorder in idiopathic normal-pressure hydrocephalus (iNPH) is predicted by right frontal hypoperfusion. However, it is not known whether bladder recovery is predicted by brain perfusion change after shunt surgery. To address this issue, we compared bladder and brain function before and after shunt surgery in iNPH.
Methods
We enrolled 75 patients in the study. Before and 12 months after shunt surgery, we analyzed brain perfusion by SPECT and bladder disorder by a specialized grading scale. The scale consisted of grade 0, none; grade 1, urinary urgency and frequency; grade 2, urinary incontinence 1–3 times a week; grade 3, urinary incontinence >daily; and grade 4, loss of bladder control. More than one grade improvement is defined as improvement, and more than one grade decrement as worsening; otherwise no changes.
Results
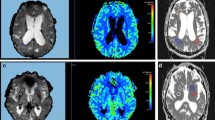
Comparing before and after surgery, in the bladder-no-change group (32 cases) there was an increase in blood flow which is regarded as reversal of enlargement in the Sylvian fissure and lateral ventricles (served as control). In contrast, in the bladder-improved group (32 cases) there was an increase in bilateral mid-cingulate, parietal, and left frontal blood flow (p < 0.05). In the bladder-worsened group (11 cases) no significant blood flow change was observed.
Conclusion
The present study showed that after shunt surgery, bladder recovery is related with mid-cingulate perfusion increase in patients with iNPH. The underlying mechanism might be functional restoration of the mid-cingulate that normally inhibits the micturition reflex.

Similar content being viewed by others
References
Hakim S, Adams RD (1965) The special clinical problem of symptomatic occult hydrocephalus with normal cerebrospinal pressure. J Neurol Sci 2:307–327
Adams RD, Fisher CM, Hakim S, Ojeman RG, Sweet WH (1965) Symptomatic occult hydrocephalus with ‘normal’ cerebrospinal pressure. N Eng J Med 273:117–126
Marmarou A, Black P, Bergsneider M, Klinge P, Relkin N, International NPH Consultant Group, International NPH Consultant Group (2005) Guidelines for management of idiopathic normal pressure hydrocephalus: progress to date. Acta Neurochir Suppl 95:237–240
Sakakibara R, Kanda T, Sekido T, Uchiyama T, Awa Y, Ito T, Liu Z, Yamamoto T, Yamanishi T, Yuasa T, Shirai K, Hattori T (2008) Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn 27:507–510
Sakakibara R, Uchida Y, Ishii K, Kazui H, Hashimoto M, Ishikawa M, Yuasa T, Kishi M, Ogawa E, Tateno F, Uchiyama T, Yamamoto T, Yamanishi T, Terada H, the members of SINPHONI (Study of Idiopathic Normal Pressure Hydrocephalus On Neurological Improvement) (2012) Correlation of right frontal hypoperfusion and urinary dysfunction in iNPH: a SPECT study. Neurourol Urodyn 31:50–55
Ishii K, Hashimoto M, Hayashida K, Hashikawa K, Chang CC, Nakagawara J, Nakayama T, Mori S, Sakakibara R (2011) A multicenter brain perfusion SPECT study evaluating idiopathic normal-pressure hydrocephalus on neurological improvement. Dement Geriatr Cogn Disord 32:1–10
Mori K (2001) Management of idiopathic normal-pressure hydrocephalus: a multi-institutional study conducted in Japan. J Neurosurg 95:970–973
Hashimoto M, Ishikawa M, Mori E, Kuwana N, Study of INPH on neurological improvement (SINPHONI) (2010) Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 7:18
Yamashita F, Sasaki M, Saito M, Mori E, Kawaguchi A, Kudo K, Natori T, Uwano I, Ito K, Saito K (2013) Voxel-based morphometry of disproportionate cerebrospinal fluid space distribution for the differential diagnosis of idiopathic normal pressurehydrocephalus. J Neuroimaging. doi:10.1111/jon.12049 [Epub ahead of print]
Sasaki H, Ishii K, Kono A, Miyamoto N, Fukuda T, Shimada K, Ohkawa S, Kawaguchi T, Mori E (2007) Cerebral perfusion pattern of idiopathic normal pressure hydrocephalus studied by SPECT and statistical brain mapping. Ann Nucl Med 21:39–45
Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE (1993) Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med 34:322–329
Minoshima S, Koeppe RA, Frey KA, Khul DE (1994) Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 35:1528–1537
Talairach J, Tounoux P (1988) Co-planar stereotaxic atlas of the human brain: an approach to medical cerebral imaging. Thieme Medical Publishers, New York
Hebb AO, Cusimano MD (2001) Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 49:1166–1184
Klinge P, Marmarou A, Bergsneider M, Relkin N, Black PM (2005) Outcome of shunting in idiopathic normal-pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery 57(3 Suppl):S40–S52
Kilic K (2010) Predicting the outcome of shunt surgery in normal pressure hydrocephalus. Neurosurgery 66:E1217
de Groat WC (2006) Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147:S25–S40
de Groat WC (2013) Lower urinary tract dysfunction: from basic science to clinical management. Foreword. Int J Urol 20:3
Sakakibara R (2014) Lower urinary tract dysfunction in patients with brain lesions. In: Vodusek D, Boller F (eds) Neurology of sexual and bladder disorders. Handbook of clinical neurology. Elsevier, Amsterdam
Andrew J, Nathan PW (1964) Lesions on the anterior frontal lobes and disturbances of micturition and defaecation. Brain 87:233–262
Kumrala E, Bayulkema G, Evyapana D, Yuntenb N (2002) Spectrum of anterior cerebral artery territory infarction: clinical and MRI findings. Eur J Neurol 9:615–624
Duffau H, Capelle L (2005) Incontinence after brain glioma surgery: new insights into the cortical control of micturition and continence. J Neurosurg 102:148–151
Fowler CJ, Griffiths DJ (2010) A decade of functional brain imaging applied to bladder control. Neurourol Urodyn 29:49–55
Jousse M, Verollet D, Guinet-Lacoste A, Le Breton F, Auclair L, G S, Amarenco S (2013) Need to void and attentional process interrelationships. BJU Int 112:E351–E357
Tadic SD, Griffiths D, Schaefer W, Resnick NM (2008) Abnormal connections in the supraspinal bladder control network in women with urgeurinary incontinence. Neuroimage 39:1647–1653
Toma AK, Stapleton S, Papadopoulos MC, Kitchen ND, Watkins LD (2011) Natural history of idiopathic normal-pressure hydrocephalus. Neurosurg Rev 34:433–439
Ouchi Y, Nakayama T, Kanno T, Yoshikawa E, Shinke T, Torizuka T (2007) In vivo presynaptic and postsynaptic striatal dopamine functions in idiopathic normal pressure hydrocephalus. J Cereb Blood Flow Metab 27:803–810
Yamamoto T, Sakakibara R, Hashimoto K, Nakazawa K, Uchiyama T, Liu Z, Ito T, Hattori T (2005) Striatal dopamine level increases in the urinary storage phase in cats: an in vivo microdialysis study. Neuroscience 135:299–303
Balaratnasingam C, Pham D, Morgan WH, Bass L, Cringle SJ, Yu DY (2009) Mitochondrial cytochrome c oxidase expression in the central nervous system is elevated at sites of pressure gradient elevation but not absolute pressure increase. J Neurosci Res 87:2973–2982
Agren-Wilsson A, Eklund A, Koskinen LO, Bergenheim AT, Malm J (2005) Brain energy metabolism and intracranial pressure in idiopathic adulthydrocephalus syndrome. J Neurol Neurosurg Psychiatry 76:1088–1093
Fan KJ, Pezeshkpour G (1987) Neurofibrillary tangles in association with congenital hydrocephalus. J Natl Med Assoc 79:1001–1003
Pickard JD, Spiegelhalter D, Czosnyka M (2006) Health economics and the search for shunt-responsive symptomatic hydrocephalus in the elderly. J Neurosurg 105:811–813 (discussion 813–814)
Author contributions
Sakakibara R has a role in study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript; Uchida Y has a role in statistical analysis of the study; Ishii K has a role in: execution of the study; Kazui H has a role in: execution of the study; Hashimoto M has a role in execution of the study; Ishikawa M has a role in execution of the study; Yuasa T has a role in execution of the study; Yamamoto T has a role in execution of the study; Yamanishi T has a role in execution of the study; Uchiyama T has a role in execution of the study; Tateno F has a role in acquisition of data; Kishi M has a role in acquisition of data; Tsuyusaki Y has a role in acquisition of data; Aiba Y has a role in execution of the study; Nagao T has a role in execution of the study; Terada H has a role in execution of the study; All the members of SINPHONI have a role in acquisition of data.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to declare relevant to this study.
Rights and permissions
About this article
Cite this article
Sakakibara, R., Uchida, Y., Ishii, K. et al. Bladder recovery relates with increased mid-cingulate perfusion after shunt surgery in idiopathic normal-pressure hydrocephalus: a single-photon emission tomography study. Int Urol Nephrol 48, 169–174 (2016). https://doi.org/10.1007/s11255-015-1162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1162-2