Abstract
Purpose
Solid-organ transplant recipients present a high rate of non-adherence to drug treatment. Few interventional studies have included approaches aimed at increasing adherence. The objective of this study was to evaluate the impact of an educational and behavioral strategy on treatment adherence of kidney transplant recipients.
Methods
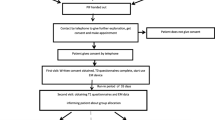
In a randomized prospective study, incident renal transplant patients (n = 111) were divided into two groups: control group (received usual transplant patient education) and treatment group (usual transplant patient education plus ten additional weekly 30-min education/counseling sessions about immunosuppressive drugs and behavioral changes). Treatment adherence was assessed using ITAS adherence questionnaire after 3 months. Renal function at 3, 6, and 12 months, and the incidence of transplant rejection were evaluated.
Results
The non-adherence rates were 46.4 and 14.5 % in the control and treatment groups (p = 0.001), respectively. The relative risk for non-adherence was 2.59 times (CI 1.38–4.88) higher in the control group. Multivariate analysis demonstrated a 5.84 times (CI 1.8–18.8, p = 0.003) higher risk of non-adherence in the control group. There were no differences in renal function and rejection rates between groups.
Conclusions
A behavioral and educational strategy addressing the patient’s perceptions and knowledge about the anti-rejection drugs significantly improved the short-term adherence to immunosuppressive therapy.
Similar content being viewed by others
References
Griva K, Ziegelmann JP, Thompson D et al (2002) Quality of life and emotional responses in cadaver and living related renal transplant recipients. Nephrol Dial Transpl 17(12):2204–2211
Lobo MCSG, Bello VAO (2007) Professional rehabilitation after kidney transplantation. J Bras Nefrol 29(1):29–32
Wolfe RA, Ashby VB, Milford EL et al (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341(23):1725–1730
McDonald S, Russ G, Campbell S, Chadban S (2007) Kidney transplant rejection in Australia and New Zealand: relationships between rejection and graft outcome. Am J Transpl 7(5):1201–1208
Meier-Kriesche HU, Schold JD, Kaplan B (2004) Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transpl 4(8):1289–1295
Sellarés J, de Freitas DG, Mengel M et al (2012) Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transpl 12(2):388–399
Butler JA, Rorerik P, Mulle MM, Masson JC, Preveler RC (2004) Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation 77(5):769–776
Denhaerynck K, Dobbels F, Cleemput I et al (2005) Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int 18(10):1121–1133
Dew MA, DiMartini AF, De Vito Dabbs A et al (2007) Rates and risk factors for non-adherence to the medical regimen after adult solid organ transplantation. Transplantation 83(7):858–873
Gordon EJ, Prohaska TR, Gallant MP, Siminoff LA (2007) Adherence to immunosuppression: a prospective diary study. Transpl Proc 39(10):3081–3085
O’Grady JG, Asderakis A, Bradley R et al (2010) Multidisciplinary insights into optimizing adherence after solid organ transplantation. Transplantation 89(5):627–632
De Bleser L, Matteson M, Dobbels F, Russell C, De Geest S (2009) Interventions to improve medication-adherence after transplantation: a systematic review. Transpl Int 22(8):780–797
Chisholm-Burns MA, Spivey CA, Graff Zivin J, Lee JK, Sredzinski E, Tolley EA (2013) Improving outcomes of renal transplant recipients with behavioral adherence contracts: a randomized controlled trial. Am J Transpl 13(9):2364–2373
De Geest S, Schäfer-Keller P, Denhaerynck K et al (2006) Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transpl 20(3):359–368
Hardstaff R, Green K, Talbot D (2003) Measurement of compliance posttransplantation—the results of a 12-month study using electronic monitoring. Transpl Proc 35(2):796–797
Russell C, Conn V, Ashbaugh C et al (2011) Taking immunosuppressive medications effectively (TIMELinK): a pilot randomized controlled trial in adult kidney transplant recipients. Clin Transpl 25(6):864–870
Chisholm MA, Lance CE, Williamson GM, Mulloy LL (2005) Development and validation of the immunosuppressant therapy adherence instrument (ITAS). Patient Educ Couns 59(1):13–20
Dobbels F, Berben L, De Geest S et al (2010) The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation 90(2):205–219
Tielen M, van Exel J, Laging M et al (2014) Attitudes to medication after kidney transplantation and their association with medication adherence and graft survival: a 2-year follow-up study. J Transpl 2014:675301. doi:10.1155/2014/675301 (Epub 2014 Apr 28)
Goldfarb-Rumyantzev AS, Wright S, Ragasa R et al (2011) Factors associated with nonadherence to medication in kidney transplant recipients. Nephron Clin Pract 117(1):33–39
Chapman JR (2004) Compliance: the patient, the doctor, and the medication? Transplantation 77(5):782–786
Bunzel B, Laederach-Hofmann K (2000) Solid organ transplantation: are there predictors for posttransplant noncompliance? A literature overview. Transplantation 70(5):711–716
Chisholm MA, Kwong WJ, Spivey CA (2007) Associations of characteristics of renal transplant recipients with clinicians’ perceptions of adherence to immnunosuppressant therapy. Transplantation 84(9):1145–1150
Chisholm-Burns MA, Spivey CA, Wilks SE (2010) Social support and immunosuppressant therapy adherence among adult renal transplant recipients. Clin Transpl 24(3):312–320
Low JK, Williams A, Manias E, Crawford K (2014) Interventions to improve medication adherence in adult kidney transplant recipients: a systematic review. Nephrol Dial Transpl 30(5):752–761
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transpl 9(Suppl 3):S1–S157. doi:10.1111/j.1600-6143.2009.02834.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Garcia MFFM declares that she has no conflict of interest; Bravin AM declares that she has no conflict of interest; Garcia PD declares that she has no conflict of interest; Nga HS declares that she has no conflict of interest; Takase HM declares that he has no conflict of interest; and Andrade LGM declares that he has no conflict of interest.
Ethical approval
All procedures performed in this work were in accordance with the ethical standards of the institutional ethics committee (under No. 3817/2011) and with the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Appendix
Appendix
Educational/interventional items and behavioral approaches addressed in treatment group
-
1.
To ensure that patients are familiar with their medications regarding their dosage, name, and reason for prescription. These points were reinforced at every consultation;
-
2.
To inform about the possible adverse effects of the medication;
-
3.
To provide written instructions in addition to the regular prescription regarding each change in dose of the drug or its frequency;
-
4.
To develop the understanding that the patient must take immunosuppressive drugs even if the transplanted organ is in good function. The team verified whether the patient was familiar with the term “rejection” and explained that a large number of patients (up to 50 %) lose the graft as a result of not taking their immunosuppressive medications properly;
-
5.
To report problems at each clinical visit and to meet the specific interests of each patient;
-
6.
To monitor the compliance with laboratory tests, clinical assessments, and medication supply;
-
7.
To use a non-judgmental approach to discuss adherence;
-
8.
To integrate medication intake with the daily routine;
-
9.
To introduce reminders, such as alarms or digital alerts;
-
10.
To attempt to produce a proactive behavior of the patient toward treatment.
Rights and permissions
About this article
Cite this article
Garcia, M.F.F.M., Bravin, A.M., Garcia, P.D. et al. Behavioral measures to reduce non-adherence in renal transplant recipients: a prospective randomized controlled trial. Int Urol Nephrol 47, 1899–1905 (2015). https://doi.org/10.1007/s11255-015-1104-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1104-z