Abstract
Purpose
The aim of this study was to evaluate the efficacy and safety of zoledronic acid (ZA) in the combination of docetaxel-based chemotherapy for castration-resistant prostate cancer with bone metastases.
Methods
We conducted a prospective study in recruiting 105 prostate cancer patients with bone metastases from 2008 to 2010. Patients were randomly divided into two groups, 53 in the docetaxel-based chemotherapy + ZA(Group A) and 52 in the docetaxel-based chemotherapy + placebo(Group B). The different outcome between patients treated with chemotherapy combined with ZA and those with chemotherapy alone was evaluated. The Cox multivariate analyses of clinical features and different treatment methods of the 105 patients were conducted.
Results
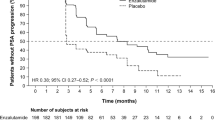
There was a response of prostate-specific antigen (PSA) in 33 (62.3 %) in Group A and 28 (53.8 %) in Group B (P = 0.20). The combined approach group had better bone progression-free survival (BPFS) (9.0 vs. 6.0 months, P < 0.05) and overall survival (OS) (19.0 vs. 15.0 months, P = 0.02), but no statistical evidence of benefit was observed in terms of PSA response. Cox multivariate analysis identified the following independent prognostic factors: received ZA, high Hb level and more than 6 cycles of chemotherapy. There were no clinical relevant differences in the frequencies of adverse events between these two groups.
Conclusions
Zoledronic acid treatment combined with docetaxel-based chemotherapy could have a better bone pain control and improve BPFS and OS for prostate cancer patients with bone metastases. The PSA response and SREs rate are similar.



Similar content being viewed by others
References
Heidenreich A, Bolla M, Joniau S et al (2009) EAU guidelines on prostate cancer. Available at: http://www.uroweb.org/nc/professional-resources/guidelines
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60(5):277–300
Stephenson AJ, Kattan MW, Eastham JA et al (2009) Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol 27(26):4300–4305
Alicikus ZA, Yamada Y, Zhang Z et al (2011) Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer 117(7):1429–1437
Dai B, Ye DW, Kong YY, Shen YJ, Wang BH (2008) Individualized prostate biopsy strategy for Chinese patients with different prostate-specific antigen levels. Asian J Androl 10:325–331
Hellerstedt BA, Pienta KJ (2002) The current state of hormonal therapy for prostate cancer. CA Cancer J Clin 52(3):154–79. doi:10.3322/canjclin.52.3.154
Diaz M, Patterson SG (2004) Management of androgen-independent prostate cancer. Cancer Control 11(6):364–373
Kaufman JM, Johnell O, Abadie E, Adami S, Audran M, Avouac B, Sedrine WB, Calvo G, Devogelaer JP, Fuchs V, Kreutz G, Nilsson P, Pols H, Ringe J, Van Haelst L, Reginster JY (2000) Back-ground for studies on the treatment of male osteoporosis: state of the art. Ann Rheum Dis 59(10):765–772
He J, Zeng ZC, Yang P, Chen B, Jiang W et al (2012) Clinical features and prognostic factors for patients with bone metastases from prostate cancer. Asian J Androl 14:505–508
Major PP, Cook R (2002) Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol 25(6 Suppl 1):S10–S18
Saad F, Gleason DM, Murray R et al (2004) Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96(11):879–882
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B (2002) Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94(19):1458–1468
Nayyar R, Sharma N, Gupta NP (2009 ) Docetaxel-based chemotherapy with zoledronic acid and prednisone in hormone refractory prostate cancer: factors predicting response and survival. Int J Urol. 16(9):726–731. doi:10.1111/j.1442-2042.2009.02351.x
Qu YY, Dai B, Kong YY, Ye DW, Yao XD, Zhang SL, Zhang HL, Ma CG, Yang WY (2013) Prognostic factors in Chinese patients with metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Asian J Androl 15(1):110–115. doi:10.1038/aja.2012.110
Tannock IF, Wit RD, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Petrylak DP, Tangen CM, Maha PH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Dagher R, Li N, Abraham S, Rahman A, Sridhara R, Pazdur R (2004) Approval summary: docetaxel in combination with prednisone for the treatment of androgen independent hormone refractory prostate cancer. Clin Cancer Res 10:147–151
DePuy V, Anstrom KJ, Castel LD, Schulman KA, Weinfurt KP, Saad F (2007) Effects of skeletal morbidities on longitudinal patient reported outcomes and survival inpatients with metastatic prostate cancer. Support Care Cancer 15(7):869–876
Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng M, Reitsma D, Urbanowitz G, Seaman JJ (2003) Zoledronic acid versus placebo in them treatment of skeletal metastases in patients with lung cancer and other solid tumors: a Phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. Clin Oncol 21(16):3150–3157
Brubaker KD, Brown LG, Vessella RL, Corey E (2006) Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer 6:15
Ullen A, Lennartsson L, Harmenberg U et al (2005) Additive/synergistic antitumoral effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncol 44:644–650
Ural AU, Avcu F (2006) Additive/synergistic anti-tumoral effects of the combination of docetaxel and zoledronic acid on prostate cancer cells: possible mechanisms? Acta Oncol 45:491–492
Sonpavde G, Pond GR, Berry WR, Wit RD, Armstrong AJ, Eisenberger MA, Tannock LF (2012) Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol 30(5):607–613. doi:10.1016/j.urolonc.2010.07.002
Smith MR, McGovern FJ, Zietman AL et al (2001) Pamidronate to prevent bone loss during androgen—deprivation therapy for prostate cancer. N Engl J Med 345:948
Wang F, Chen W, Chen H, Mo L, Jin H, Yu Z, Li C, Liu Q, Duan F, Weng Z (2013) Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Med Oncol 30(3):657. doi:10.1007/s12032-013-0657-x
Schiavenato M, Craig KD (2010) Pain assessment as a social transaction: beyond the “gold standard”. Clin J Pain 26(8):667–676
Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, Small EJ (2008) Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 26:2544–2549
Yee CH, Ng CF, Wong AY, Chan CK, Hou SM, Yip SK (2011) Zoledronic acid to prevent bone loss in Chinese men receiving androgen deprivation therapy for prostate cancer. Asia Pac J Clin Oncol 7:168–173. doi:10.1111/j.1743-7563.2011.01388.x
Shamseddine A1, Farhat FS, Elias E, Khauli RB, Saleh A, Bulbul MA (2013) High-dose calcitriol, docetaxel and zoledronic acid in patients with castration-resistant prostate cancer: a phase II study. Urol Int 90(1):56–61. doi:10.1159/000343780
Sabry NA, Habib EE (2011) Zoledronic acid and clodronate in the treatment of malignant bone metastases with hypercalcaemia; efficacy and safety comparative study. Med Oncol 28(2):584–590. doi:10.1007/s12032-010-9461-z
Acknowledgments
This study was funded by the Wenzhou science bureau project (Y20100023). We thank lecturer Fengsu. Yang for her generous help in manuscript revision. This work or part of results has not been published in any other journal or publication.
Conflict of interest
All authors state that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yue Pan and Haiyong Jin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pan, Y., Jin, H., Chen, W. et al. Docetaxel with or without zoledronic acid for castration-resistant prostate cancer. Int Urol Nephrol 46, 2319–2326 (2014). https://doi.org/10.1007/s11255-014-0824-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0824-9