Abstract
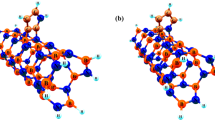
Boron nitride nanotube (BNNT) as an innovative support for carbohydrate transformation processes was evaluated, using density functional theory. The α-d-glucopyranose adsorption on a Pd30 cluster, supported on BNNT, was used to check both the local activity of topologically different metallic sites and the effects of the proximity of the BNNT surface to the same metallic sites. Detailed geometrical and electronic analyses performed on Pd30/BNNT and α-d-glucopyranose/Pd30/BNNT systems were discussed. It was observed that the deposition of the Pd30 cluster onto the BNNT support gives rise to an electronic rearrangement, determining a charge transfer from the support to the adsorbed metal cluster. The charge transfer, as shown by the analysis of molecular electrostatic potential, seems to generate electron-rich and electron-poor zones in the Pd30 cluster. The α-d-glucopyranose species, regardless the interaction geometry experienced, acts as an electron donor and preferentially adsorbs close to the electron-poor metal/support interface.




Similar content being viewed by others
References
Armaković SJ, Armaković S, Finĕur NL, Šibul F, Vione D, Šetrajčić JP, Abramović BF (2015) Influence of electron acceptors on the kinetics of metoprolol photocatalytic degradation in TiO2 suspension: a combined experimental and theoretical study. RSC Adv 5:54589–54604
Becton M, Wang X (2015) Grain-size dependence of mechanical properties in polycrystalline boron–nitride: a computational study. Phys Chem Chem Phys 17:21894–21901
Besson M, Gallezot P, Pinel C (2014) Conversion of biomass into chemicals over metal catalysts. Chem Rev 114:1827–1870
Bruix A, Rodriguez JA, Ramìrez PJ, Senanayake SD, Evans J, Park JB, Stacchiola D, Liu P, Hrbek J, Illas F (2012) A new type of strong metal-support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) catalysts. J Am Chem Soc 134:8968–8974
Campbell CT (2012) Catalyst-support interactions: electronic perturbations. Nat Chem 4:597–598
Corchado JC, Sànchez ML, Aguilar MA (2004) Theoretical study of the relative stability of rotational conformers of α and β-d-glucopyranose in gas phase and aqueous solution. J Am Chem Soc 126:7311–7319
Cortese R, Duca D, Sifontes Herrera VA, Murzin DY (2012) l-arabinose conformers adsorption on ruthenium surfaces: a DFT study. J Phys Chem C 116:14908–14916
Cortese R, Schimmenti R, Armata N, Ferrante F, Prestianni A, Duca D, Murzin DY (2015) Investigation of polyol adsorption on Ru, Pd, and Re using vdW density functionals. J Phys Chem C 119:17182–17192
Cramer CJ, Truhlar DG (1993) Quantum chemical conformational analysis of glucose in aqueous solution. J Am Chem Soc 115:5745–5753
Crespo-Quesada M, Yoon S, Jin M, Prestianni A, Cortese R, Càrdenas-Lizana F, Duca D, Weidenkaff A, Kiwi-Minsker L (2015) Shape-dependence of Pd nanocrystal carburization during acetylene hydrogenation. J Phys Chem C 119:1101–1107
Dapprich S, Komàromi I, Byun KS, Morokuma K, Frisch MJ (1999) A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J Mol Struct THEOCHEM 461–462:1–21
Delidovich IV, Taran OP, Matvienko LG, Simonov AN, Simakova IL, Bobrovskaya AN, Parmon VN (2010) Selective oxidation of glucose over carbon-supported Pd and Pt catalysts. Catal Lett 140:14–21
Duca D, Ferrante F, La Manna G (2007) Theoretical study of palladium cluster structures on carbonaceous supports. J Phys Chem C 111:5402–5408
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheese-man JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian09 revision D.01. Gaussian Inc., Wallingford
Gao N, Fang X (2015) Synthesis and development of graphene–inorganic semiconductor nanocomposites. Chem Rev 115(16):8294–8343
Hermans S, Diverchy C, Dubois V, Devillers M (2014) Pd nanoparticles prepared by grafting of Pd complexes on phenol-functionalized carbon supports for liquid phase catalytic applications. Appl Catal A 474:263–271
Kacprzak KA, Czekaj I, Mantzaras J (2012) DFT studies of oxidation routes for Pd9 clusters supported on γ-alumina. Phys Chem Chem Phys 14:10243–10247
Kim KS, Kingston CT, Hrdina A, Jakubinek MB, Guan J, Plunkett M, Simard B (2014) Hydrogen-catalyzed, pilot-scale production of small-diameter boron nitride nanotubes and their macroscopic assemblies. ACS Nano 8:6211–6220
Koitz R, Norskov JK, Studt F (2015) A systematic study of metal-supported boron nitride materials for the oxygen reduction reaction. Phys Chem Chem Phys 17:12722–12727
Lin CA, Wu JCS, Pan JW, Yeh CT (2002) Characterization of boron–nitride-supported Pt catalysts for the deep oxidation of benzene. J Catal 210:39–45
Mager N, Meyer N, Léonard AF, Job N, Devillers M, Hermans S (2014) Functionalization of carbon xerogels for the preparation of palladium supported catalysts applied in sugar transformations. Appl Catal B 148–149:424–435
Meyer N, Bekaert K, Pirson D, Devillers M, Hermans S (2012) Boron nitride as an alternative support of Pd catalysts for the selective oxidation of lactose. Catal Commun 29:170–174
Meyer N, Pirson D, Devillers M, Hermans S (2013) Particle size effects in selective oxidation of lactose with Pd/h-BN catalysts. Appl Catal A 467:463–473
Meyer N, Devillers M, Hermans S (2015) Boron nitride supported Pd catalysts for the hydrogenation of lactose. Catal Today B 241:200–207
Murzin DY (1995) On the kinetic interpretation of metal-support interaction. React Kinet Catal Lett 55:275–281
Pacchioni G (2013) Electronic interactions and charge transfers of metal atoms and clusters on oxide surfaces. Phys Chem Chem Phys 15:1737–1757
Postole G, Gervasini A, Guimon C, Auroux A, Bonnetot B (2006) Influence of the preparation method on the surface characteristics and activity of boron–nitride-supported noble metal catalysts. J Phys Chem B 110:12572–12580
Prestianni A, Ferrante F, Simakova OA, Duca D, Murzin DY (2013) Oxygen-assisted hydroxymatairesinol dehydrogenation: a selective secondary-alcohol oxidation over a gold catalyst. Chem Eur J 19:4577–4585
Prestianni A, Crespo-Quesada M, Cortese R, Ferrante F, Kiwi-Minsker L, Duca D (2014) Structure sensitivity of 2-methyl-3-butyn-2-ol hydrogenation on Pd: computational and experimental modeling. J Phys Chem C 118:3119–3128
Prestianni A, Ferrante F, Sulman EM, Duca D (2014) Density functional theory investigation on the nucleation and growth of small palladium clusters on a hyper-cross-linked polystyrene matrix. J Phys Chem C 118:21006–21013
Rappe AK, Casewit CJ, Colwell KS, Goddard WA, Skiff WM (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:10024–10035
Schimmenti R, Cortese R, Ferrante F, Prestianni A, Duca D (2016) Growth of sub-nanometric palladium clusters on boron nitride nanotubes: a DFT study. Phys Chem Chem Phys 18:1750–1757
Tauster SJ (1987) Strong metal-support interactions. Acc Chem Res 20:389–394
Terrones M, Romo-Herrera JM, Cruz-Silva E, Lòpez-Urìas F, Munõz- Sandoval E, Velàzquez-Salazar JJ, Terrones H, Bando Y, Golberg D (2007) Pure and doped boron nitride nanotubes. Mater Today 10:30–38
Tokarev AV, Murzina EV, Kuusisto J, Mikkola J-P, Eranen K, Murzin DY (2006) Kinetic behaviour of electrochemical potential in three- phase heterogeneous catalytic oxidation reactions. J Mol Catal A 255:199–208
Venezia AM, Duca D, Floriano MA, Deganello G, Rossi A (1992) Chemical effect on the XPS spectra of the valence band and on O KLL and Pd MNN Auger spectra in pumice-supported catalysts. Surf Interface Anal 18:619–622
Wang YG, Yoon Y, Glezakou VA, Li J, Rousseau R (2013) The role of reducible oxide-metal cluster charge transfer in catalytic processes: new insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J Am Chem Soc 135:10673–10683
Willinger MG, Zhang W, Bondarchuk O, Shaikhutdinov S, Freund HJ, Schlögl R (2014) A case of strong metal-support interactions: combining advanced microscopy and model systems to elucidate the atomic structure of interfaces. Angew Chem Int Ed 53:5998–6001
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM–B3LYP). Chem Phys Lett 393:51–57
Zhang J, Alexandrova AN (2012) Double σ–aromaticity in a surface-deposited cluster: Pd4 on TiO2 (110). J Phys Chem Lett 3:751–754
Zhang L, Wu P, Sullivan M (2011) Hydrogen adsorption on Rh, Ni, and Pd functionalized single-walled boron nitride nanotubes. J Phys Chem C 115:4289–4296
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prestianni, A., Cortese, R., Ferrante, F. et al. α-d-Glucopyranose Adsorption on a Pd30 Cluster Supported on Boron Nitride Nanotube. Top Catal 59, 1178–1184 (2016). https://doi.org/10.1007/s11244-016-0638-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-016-0638-3