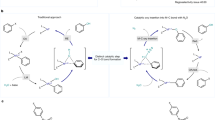
Abstract
In this contribution we use computational tools to investigate the reaction of alcohol substrates with reactive nitrogen oxide species such as N2O3 and N2O4, leading to the formation of alkyl nitrites. These nitrites are interesting intermediates which can be processed to various valuable chemicals such as ketones/aldehydes and dimethyl oxalate while regenerating NO x . As such, NO x is used as an oxidation mediator, converting alcohol substrates to more reactive nitrites which can be selectively converted to more desired compounds, closing a catalytic cycle in NO x species.










Similar content being viewed by others
References
Rebsdat S, Mayer D (2011) Ethylene Oxide, Ullmann’s Encyclopedia of Industrial Chemistry. Wiley, Weinheim
Umemura S, Miyazaki H (1984) Kagaku Kogyo 35:34
Uchiumi SI, Ataka K, Matsuzaki T (1999) J Organomet Chem 576:279
Uchiumi SI, Ataka K (2002) Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley, New York, pp 2691–2704
Fang D, Ying W (1993) Huaxue Shijie 34:583
He L, Xiao H, Li Y (2006) Gongye Cuihua 14:11
Wu L (2008) Shanghai Huagong 33:18
Ma Z, Meng X, Wang H, Zhou W, Jiang H (2009) Shiyou Huagong 38:456
Zhou J, Liu X, Liu D (2009) Huagong Jinzhan 28:47
Wang J, Yang W, Lu J (2009) Huagong Jinzhan 28:1216
Boswell C (2012) ICIS Chem Bus 281(5):32–33
Hantzsch A (1901) Chem Ber 36:2097
Langenbeck W, Richter M (1956) Chem Ber 89:202
Niki H, Maker PD, Savage CM, Breitenbach LP (1982) Intern J Kinetics 14:1199
Koda S, Yoshikawa K, Okada J, Akita K (1985) Environ Sci Technol 19:262
Liu G, Ji Y, Li W (2010) Chem Eng J 157:483
Wang H, Li G (2010) Chem Eng J 163:422
Ji Y, Zhang B, Liu G, Li W, Xiao W (2010) Tianranqi Huagong 35:12
(a) US 4353843 and references therein; (b) US2831882; (c) DE1156775; (d) US1691302; (e) US4467109; (f) US 4229589; (g) EP1346976; (h) CN102008922; (i) CN201711149
Aellig C, Neuenschwander U, Hermans I (2012) ChemCatChem 4:525
Aellig C, Girard C, Hermans I (2011) Angew Chem Int Ed 50:12355
Aellig C, Scholz D, Hermans I (2012) ChemSusChem 9:1732
Liu Y-D, Zhong RG (2010) Chin J Str Chem 29(3):421
Sun Z, Liu YD, Lv CL, Zhong RG (2009) J Mol Structure 908:107
Liu W-G, Goddard WA (2012) J Am Chem Soc 134:12970
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers EE, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma R, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian Inc., Wallingford
Becke AD (1992) J. Chem. Phys. 96:2155
Becke AD (1992) J Chem Phys. 97:9173
Becke AD (1993) J Chem Phys. 98:5648
Chai JD, Head-Gordon M (2008) J Chem Phys. 128:084106
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys. 87:5968
Ochterski JW, Petersson GA, Montgomery JA Jr (1996) J Chem Phys. 104:2598
Bartlett RJ, Purvis GD III (1978) Int J Quantum Chem 14:561
Pople JA, Krishnan R, Schlegel HB, Binkley JS (1978) Int J Quantum Chem 14:545
Varetti EL, Pimentel GC (1971) J Chem Phys. 55:3813
Nour EM, Chen LH, Laane J (1983) J Phys Chem 87:1113
Holland RF, Maier WB, Ii J (1983) Chem Phys 78:2928
Hermans I, Nguyen TL, Jacobs PA, Peeters J (2005) ChemPhysChem 6:637
Albright TA, Burdett JK, Whangbo M-H (1985) Orbital Interactions in Chemistry. Wiley, Hoboken
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999
Acknowledgments
IH acknowledges financial support from the ETH Zurich and the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermans, I., Teles, J.H., Dehn, R. et al. Formation Mechanism of Alkyl Nitrites, Valuable Intermediates in C1-Upgrading Chemistry and Oxidation Processes. Top Catal 57, 1256–1264 (2014). https://doi.org/10.1007/s11244-014-0291-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-014-0291-7