Abstract
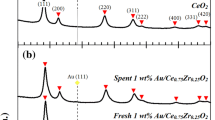
As traditional sources of energy become depleted, significant research interest has gone into conversion of biomass into renewable fuels. Biomass-derived synthesis gas typically contains concentrations ranging from ~30 to 600 ppm H2S. H2S is a catalyst poison which adversely impacts downstream processing of hydrogen for gas-to-liquid plants and the deactivation of water–gas shift catalysts by sulfur is typical. Novel catalysts are needed to remain active in the presence of sulfur in order to boost efficiency and mitigate costs. Previous studies have shown molybdenum to be active in concentrations of sulfur >300 ppm. Cobalt has been shown to be active as a spinel in concentrations of sulfur <240 ppm. Ceria has received attention as a catalyst due to its oxygen donating properties. In this study, mixed oxide catalysts were synthesized via Pechini’s method into various blends of metal oxide solutions. Activity testing at low steam-to-carbon ratios (1:1) produced near equilibrium conversions at a GHSV of 6,300 h−1 and over a temperature range of 350–400 °C for a Ce–Co mixed oxide even after an 800 ppm sulfur treatment. The addition of molybdenum to the Ce–Co base had little effect on sulfur tolerance, but it did lead to a reduction in selectivity for methanation. Specific surface areas generally increased following the sulfur treatments and X-ray diffraction patterns confirmed that bulk sulfiding did not occur.





Similar content being viewed by others
References
Mond L, Langer C (1888) British Patent 12608
Armor JN (1999) The multiple roles for catalysis in the production of H2. Appl Catal A 176(2):159–176
Armor JN (2005) Catalysis and the hydrogen economy. Catal Lett 101(3):131–135
Ladebeck JR, Wagner JP (2010) Catalyst development for water–gas shift. In: Handbook of fuel cells. Wiley, Chichester
Song C (2003) Overview of hydrogen production options for hydrogen energy development, fuel-cell fuel processing and mitigation of CO2 emissions. In Proc. 20th International Pittsburgh Coal Conference, National Science Foundation, Pittsburgh
Bartholomew CH, Agrawal PK, Katzer JR (1982) Sulfur Poisoning of Metals. In: Eley DDHP, Paul BW (eds) Advances in catalysis, vol 31. Academic Press, New York, pp 135–242
Yung MM, Jablonski WS, Magrini-Bair KA (2009) Review of catalytic conditioning of biomass-derived syngas. Energy & Fuels 23(4):1874–1887
Kuhn JN, Lakshminarayanan N, Ozkan US (2008) Effect of hydrogen sulfide on the catalytic activity of Ni-YSZ cermets. J Mol Catal A 282:9–21
Yung MM, Kuhn JN (2010) Deactivation mechanisms of Ni-based tar reforming catalysts as monitored by X-ray absorption spectroscopy. Lang 26:16589–16594
Dry ME (2002) The Fischer–Tropsch process: 1950–2000. Catal Today 71(3–4):227–241
Song C (2002) Fuel processing for low-temperature and high-temperature fuel cells: challenges, and opportunities for sustainable development in the 21st century. Catal Today 77(1–2):17–49
Petrov K, Srinivasan S (1996) Low temperature removal of hydrogen sulfide from sour gas and its utilization for hydrogen and sulfur production. Int J Hydrogen En 21(3):163–169
Yung MM, Cheah S, Magrini-Bair KA, Kuhn JN (2012) Transformation of sulfur species during steam/air regeneration on a Ni biomass conditioning catalyst. ACS Catal 2:1363–1367
Newsome DS (1980) The water–gas shift reaction. Catal Rev 21(2):275–318
Ratnasamy C, Wagner JP (2009) Water gas shift catalysis. Catal Rev 51(3):325–440
Zhang L, Millet J-MM, Ozkan US (2009) Deactivation characteristics of Fe–Al–Cu water–gas shift catalysts in the presence of H2S. J Mol Catal A 309(1–2):63–70
Hakkarainen R, Salmi T, Keiski RL (1993) Water–gas shift reaction on a cobalt-molybdenum oxide catalyst. Appl Catal A 99(2):195–215
Ratnasamy P, Sivasanker S (1980) Structural chemistry of Co–Mo–Alumnina Catalysts. Catal Rev 22(3):401–429
Cheah S, Carpenter DL, Magrini-Bair KA (2009) Review of mid- to high-temperature sulfur sorbents for desulfurization of biomass- and coal-derived syngas. En & Fuels 23(11):5291–5307
de la Osa AR, De Lucas A, Valverde JL, Romero A, Monteagudo I, Sánchez P (2011) Performance of a sulfur-resistant commercial WGS catalyst employing industrial coal-derived syngas feed. Int J Hydrogen Energy 36(1):44–51
Liu B, Goldbach A, Xu H (2011) Sour water–gas shift reaction over Pt/CeO2 catalysts. Catal Today 171(1):304–311
Andreev AA, Kafedjiysky VJ, Edreva-Kardjieva RM (1999) Active forms for water–gas shift reaction on NiMo-sulfide catalysts. Appl Catal A 179(1–2):223–228
Nikolova D, Edreva-Kardjieva R, Grozeva T (2011) Water–gas shift activity of K-promoted (Ni)Mo/γ-Al2O3 systems in sulfur-containing feed. React Kin Mech Catal 103(1):71–86
Wang H, Lian Y, Zhang Q, Li Q, Fang W, Yang Y (2008) MgO–Al2O3 mixed oxides-supported Co–Mo-based catalysts for high-temperature water–gas shift reaction. Catal Lett 126(1):100–105
Lian Y, Xiao R, Fang W, Yang Y (2011) Potassium-decorated active carbon supported Co–Mo-based catalyst for water–gas shift reaction. J Nat Gas Chem 20(1):77–83
Copperthwaite RG, Gottschalk FM, Sangiorgio T, Hutchings GJ (1990) Cobalt chromium oxide: a novel sulphur tolerant water–gas shift catalyst. Appl Catal 63(1):L11–L16
Mellor JR, Copperthwaite RG, Coville NJ (1997) The selective influence of sulfur on the performance of novel cobalt-based water–gas shift catalysts. Appl Catal A 164(1–2):69–79
Hou P, Meeker D, Wise H (1983) Kinetic studies with a sulfur-tolerant water gas shift catalyst. J Catal 80(2):280–285
Hutchings GJ, Copperthwaitet RG, Gottschalk FM, Hunter R, Mellor J, Orchard SW, Sangiorgio T (1992) A comparative evaluation of cobalt chromium oxide, cobalt manganese oxide, and copper manganese oxide as catalysts for the water–gas shift reaction. J Catal 137(2):408–422
Laniecki M, Zmierczak W (1991) Deactivation of sulfur tolerant water–gas shift catalysts based on Ni–Mo–Y–zeolites. In: Calvin HB, John BB (eds) Studies in surface science and catalysis, vol 68. Elsevier, Amsterdam, pp 799–802
Yates IC, Satterfield CN (1991) Intrinsic kinetics of the Fischer–Tropsch synthesis on a cobalt catalyst. Energy & Fuels 5(1):168–173
Van Der Laan GP, Beenackers AACM (1999) Kinetics and selectivity of the Fischer–Tropsch synthesis: a literature review. Catal Rev 41(3–4):255–318
Gottschalk FM, Hutchings GJ (1989) Manganese oxide water–gas shift catalysts initial optimization studies. Appl Catal 51(1):127–139
Fornasari G, Gusi S, Trifiro F, Vaccari A (1987) Cobalt mixed spinels as catalysts for the synthesis of hydrocarbons. Ind Eng Chem Res 26(8):1500–1505
Lausche AC, Schaidle JA, Thompson LT (2011) Understanding the effects of sulfur on Mo2C and Pt/Mo2C catalysts: methanol steam reforming. Appl Catal A 401(1–2):29–36
Laniecki M, Ignacik M (2006) Water–gas shift reaction over sulfided molybdenum catalysts supported on TiO2–ZrO2 mixed oxides support characterization and catalytic activity. Catal Today 116(3):400–407
Valsamakis I, Flytzani-Stephanopoulos M (2011) Sulfur-tolerant lanthanide oxysulfide catalysts for the high-temperature water–gas shift reaction. Appl Catal B 106(1–2):255–263
Xue E, O’Keeffe M, Ross JRH (2000) A study of Pt/ZrO2 catalysts for water–gas shift reaction in the presence of H2S. In: Avelino Corma FVMSM, José Luis GF (eds) Studies in surface science and catalysis, vol 130. Elsevier, Amsterdam, pp 3813–3818
Bunluesin T, Gorte RJ, Graham GW (1998) Studies of the water–gas-shift reaction on ceria-supported Pt, Pd, and Rh: implications for oxygen-storage properties. Appl Catal B 15(1–2):107–114
Gorte RJ (2010) Ceria in catalysis: from automotive applications to the water–gas shift reaction. AIChE J 56(5):1126–1135
Mogensen M, Sammes NM, Tompsett GA (2000) Physical, chemical and electrochemical properties of pure and doped ceria. Sol Stat Ion 129(1–4):63–94
Trovarelli A (1996) Catalytic properties of ceria and CeO2-containing materials. Catal Rev 38(4):439–520
Tabakova T, Boccuzzi F, Manzoli M, Andreeva D (2003) FTIR study of low-temperature water–gas shift reaction on gold/ceria catalyst. Appl Catal A 252(2):385–397
Li Y, Fu Q, Flytzani-Stephanopoulos M (2000) Low-temperature water–gas shift reaction over Cu- and Ni-loaded cerium oxide catalysts. Appl Catal B 27(3):179–191
Jacobs G, Patterson PM, Williams L, Chenu E, Sparks D, Thomas G, Davis BH (2004) Water–gas shift: in situ spectroscopic studies of noble metal promoted ceria catalysts for CO removal in fuel cell reformers and mechanistic implications. Appl Catal A 262(2):177–187
Reddy GK, Boolchand P, Smirniotis PG (2011) Sulfur tolerant metal doped Fe/Ce catalysts for high temperature WGS reaction at low steam to CO ratios—XPS and Mössbauer spectroscopic study. J Catal 282(2):258–269
Moe JM (1962) Design of water–gas shift reactors. Chem Eng Prog 58:33–36
Carbo MC, Boon J, Jansen D, van Dijk HAJ, Dijkstra JW, van den Brink RW, Verkooijen AHM (2009) Steam demand reduction of water–gas shift reaction in IGCC power plants with pre-combustion CO2 capture. Int J Green Gas Control 3(6):712–719
Liu B, Zong Q, Edwards PP, Zou F, Du X, Jiang Z, Xiao T, AlMegren H (2012) Effect of titania addition on the performance of CoMo/Al2O3 sour water gas shift catalysts under lean steam to gas ratio conditions. Ind Eng Chem Res 51(36):11674–11680
Prins R, De Beer VHJ, Somorjai GA (1989) Structure and function of the catalyst and the promoter in Co–Mo hydrodesulfurization catalysts. Catal Rev 31(1–2):1–41
Pechini M (1967) US Patent 3,330,697
Barroso MN, Gomez MF, Gamboa JA, Arrúa LA, Abello MC (2006) Preparation and characterization of CuZnAl catalysts by citrate gel process. J Phys Chem Solid 67(7):1583–1589
Caldwell TE, Abdelrehim IM, Land DP (1996) Thiophene decomposition on Pd(111) forms S and C4 species: a laser-induced thermal desorption/Fourier transform mass spectrometry study. Surf Sci 367(1):L26–L31
Simon LJ, Rep M, van Ommen JG, Lercher JA (2001) Thiophene decomposition on Pt-supported zeolites: a TPD study. Appl Catal A 218(1–2):161–170
Babich IV, Moulijn JA (2003) Science and technology of novel processes for deep desulfurization of oil refinery streams: a review. Fuel 82(6):607–631
Bligaard T, Nørskov JK, Dahl S, Matthiesen J, Christensen CH, Sehested J (2004) The Brønsted–Evans–polanyi relation and the volcano curve in heterogeneous catalysis. J Catal 224(1):206–217
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319
Saito M, Anderson RB (1980) The activity of several molybdenum compounds for the methanation of CO. J Catal 63(2):438–446
Acknowledgments
The authors thank Umit S. Ozkan for her many contributions to the fields of heterogeneous catalysis and energy and fuels. The authors gratefully acknowledge funding from the National Science Foundation contract number 1046518.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roberge, T.M., Blavo, S.O., Holt, C. et al. Effect of Molybdenum on the Sulfur-Tolerance of Cerium–Cobalt Mixed Oxide Water–Gas Shift Catalysts. Top Catal 56, 1892–1898 (2013). https://doi.org/10.1007/s11244-013-0125-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0125-z